In 1989, SONY company found that petroleum coke carbon materials could be used to replace metal lithium to make secondary batteries, which really opened the prelude to the large-scale application of lithium-ion batteries. In the next 30 years, three generations of products such as carbon,
lithium titanate and silicon-based materials have been used as anode materials. This article will briefly introduce various lithium-ion battery anode materials according to the structural classification of anode materials.
The ideal anode material should have the following characteristics:
1.Low lithium insertion potential: To ensure a higher output voltage (the potential of the lithium ion embedded in the negative electrode material relative to the reference electrode (standard lithium electrode), which is an important parameter in the discharge process of the battery);
2.High theoretical specific capacity: Allow more lithium ion reversible de/intercalation (specific capacity is an indicator to measure the capacity of the battery to store energy, it represents the unit mass or unit volume of the battery (active material) can release the amount of electricity);
3.Long cycle life: The structure is relatively stable during the charge and discharge process (cycle life is one of the important indicators to measure the performance of the battery, which refers to the number of charge and discharge cycles that the battery can withstand before the capacity decays to a specified value);
4.Excellent rate performance: High lithium ion diffusion coefficient, high diffusion rate inside and on the surface of the electrode material;
5.Good conductivity: including electronic conductivity, ionic conductivity and low charge transfer resistance to ensure smaller voltage polarization and good rate performance (conductivity is a physical quantity describing the conductivity of the material, which indicates the ease of flow of current in the material);
6.Stable solid electrolyte membrane: A stable interface with the electrolyte is formed to ensure high coulombic efficiency.
The anode material of lithium-ion battery is one of the key factors of battery performance, which directly affects the key parameters such as energy density, power density, cycle life and safety of the battery. Up to now, the common anode materials are mainly divided into carbon-based anode, oxide-based anode, phosphorus-based anode, silicon-based anode and other anode materials.
1.Carbon-based anodes: Carbon-based anode materials are the most common anode materials, which are mainly divided into graphite and amorphous carbon.
(1)Graphite: Graphite materials are mainly divided into natural graphite and artificial graphite:
I.Natural graphite: Natural graphite can be divided into flake graphite and soil-like graphite, and the negative electrode material is usually flake graphite, which has large reserves, low cost, low potential and smooth curve. In the appropriate electrolyte, the first cycle coulombic efficiency is 90%~93%, and the reversible capacity can reach 340~370 mAh·g-1. The structure diagram is shown in Figure 1. However, the regular layered structure of natural graphite leads to its high anisotropy, resulting in the slow insertion of lithium ions and insufficient contact between graphite particles and current collector, which is also the main reason for the low rate performance of natural graphite.
II.Artificial graphite: Artificial graphite is the graphite material prepared by calcining easily graphitized carbon (petroleum coke, needle coke, asphalt, etc.) at a certain temperature, and then crushing, molding, grading, and high-temperature graphitization. After continuous modification research, artificial graphite has approached or even surpassed natural graphite in terms of capacity, first-cycle efficiency, and cycle life, but high-temperature graphitization also brings high cost defects.
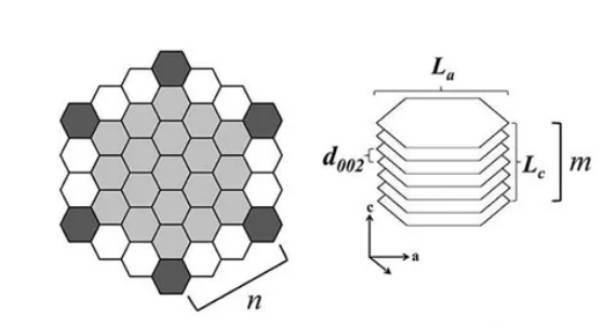
Figure-1-The-structure-diagram-of-graphite
(2)Amorphous carbon: Amorphous carbon materials are mainly divided into hard carbon and soft carbon:
I.Soft carbon: Soft carbon is carbon that is easily graphitized by high temperature treatment (above 2500 °C). Compared with graphite, soft carbon has the characteristics of large specific surface area, stable crystal structure and strong electrolyte adaptability. Due to avoiding graphitization treatment, the cost of soft carbon materials is lower. The capacity of soft carbon materials is generally 200~250 mAh·g-1, and the cycle performance can be improved to more than 1500 times.
II.Hard carbon: Hard carbon refers to carbon that is difficult to be graphitized under high temperature treatment (above 2500 °C). Its structure is disordered, and there are few layers of graphite sheets and many defects. Compared with graphite, the solvent co-intercalation and significant lattice expansion and contraction of hard carbon do not occur, which has good cycle performance. The specific capacity of hard carbon can reach 400~600 mAh·g-1 without the limitation of lithium intercalation potential.
2.Oxide-based anodes: such as tin oxide, lithium titanate, etc., these materials have advantages and disadvantages compared to carbon-based anodes.
(1)Tin oxide: Tin oxide (SnO2) as an anode material for lithium-ion batteries has the advantage of high theoretical specific capacity, reaching 782 mAh·g-1, which makes it theoretically able to provide higher energy density than traditional carbon materials. In addition to high theoretical specific capacity, tin-based anode materials also have the characteristics of environmental friendliness, high safety and low cost. These properties make tin-based materials considered to be one of the most promising alternative anode materials for next-generation lithium-ion batteries.
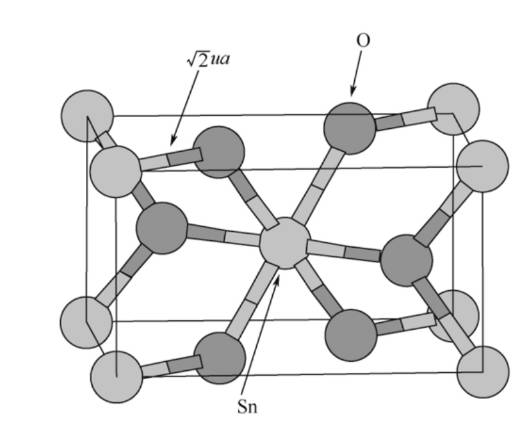
Figure-2-The-structure-diagram-of-SnO2
(2)Lithium titanate: Lithium titanate material is considered to be one of the most promising anode materials due to its high safety, long life and low strain. The structure diagram is shown in Figure 3. However, the theoretical capacity and intrinsic conductivity of lithium titanate are low, which limits its large-scale application. In order to improve these problems, researchers are exploring various modification methods, such as ion doping, carbon surface modification and nanocrystallization.
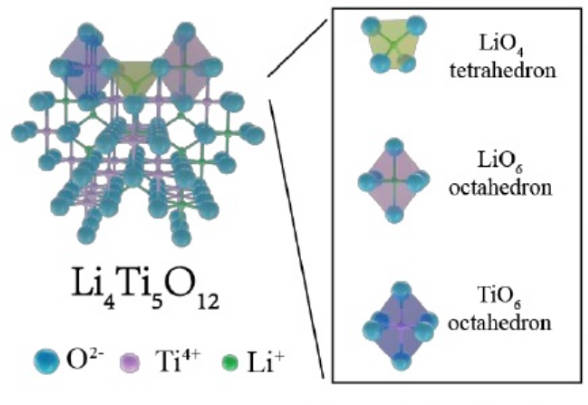
Figure-3-The-structure-diagram-of-Li4Ti5O12
3.Phosphorus-based anodes: Phosphorus-based materials have attracted much attention due to their high theoretical capacity, but they may encounter problems of volume expansion and cycle stability in practical applications. The following characteristics of the material limit its further application:
(1)Poor conductivity: The conductivity of some phosphorus-based materials is low, which limits their electrochemical performance and rate capability;
(2)Large volume change: During the charge-discharge cycle, the phosphorus-based material will undergo a large volume expansion, which may lead to the destruction of the material structure and the decrease of the cycle stability;
(3)Low lithium storage efficiency: The main reasons for the low lithium storage efficiency of phosphorus-based anode materials include poor conductivity, large volume change, interface instability and oxidation problems. The methods to improve the lithium storage efficiency of phosphorus-based anode materials include nanocrystallization treatment, morphology design and composite material preparation.
4.Silicon-based anode: Silicon has a very high theoretical specific capacity, which is several times that of carbon materials, but there are still problems such as volume expansion during charging and discharging. It mainly has the following characteristics:
(1)High specific capacity: The biggest advantage of silicon-based anode materials lies in their high specific capacity, which means that they can store more energy under the same volume or weight, which is crucial for improving the energy density of the battery and prolonging the service life of the battery;
(2)Volume expansion problem: During the charge and discharge process, the silicon-based anode will undergo volume expansion, which will lead to battery capacity attenuation and coulombic efficiency decline, affecting the cycle stability and service life of the battery. The schematic diagram is shown in Figure 4;
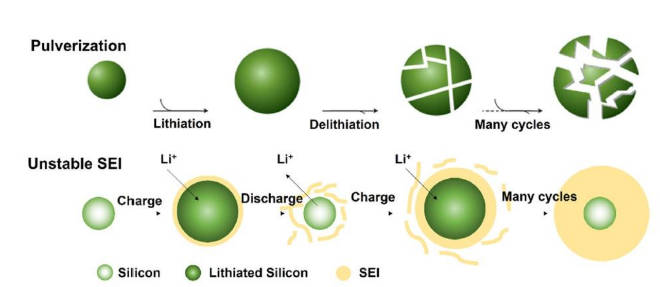
Figure-4-The-volume-expansion-diagram-of-silicon-based-anode
(3)Poor conductivity: Compared with carbon materials, silicon-based anodes have poor conductivity, which may affect the charge-discharge efficiency and power output of the battery.
In order to solve the problems faced by silicon-based anodes, researchers are exploring a variety of improvement methods, including prelithiation, dry electrode technology, supporting improvement, synthesis technology and structural design.
5.Other anode materials: In addition to the above four types of anode materials, there are still new organic materials and other materials in the field of anode materials for lithium ion batteries. In the future, more materials will be explored for the characteristics of the above anode materials.
Research topics of anode materials of lithium-ion batteries
In the future, lithium ion battery anode materials may develop in the following directions:
1.Through material design and synthesis of new carbon-based materials to improve their lithium storage capacity and reduce the preparation cost of materials. Researchers will also continue to explore the relationship between the microstructure and electrochemical properties of carbon materials, and find out the mechanism of ferroelectricity affecting the electrochemical behavior of carbon materials;
2.The preparation technology of silicon-based anode materials will be further developed, and the cycle stability and rate performance of silicon materials will be improved by means of nanostructure design and surface coating. The composite application of silicon-based materials and other materials will also be explored to expand the application range of silicon materials in lithium batteries;
3.The research on metal oxides will also continue to deepen in order to find new metal oxide materials and improve their structure and properties. The researchers will also further study the embedding / deintercalation mechanism of metal oxides to solve the problem of cycle stability.




The lab focuses on solid-state battery research to overcome traditional lithium batteries' safety and energy density issues, supporting environmental sustainability. It develops innovative solid-state electrolytes, refines electrode materials, and investigates ion transfer and interface stability to revolutionize battery technology.

The electric vehicle battery industry is rapidly developing, focusing on technological innovation, market competition, and sustainability. Research hotspots include solid-state batteries, new types of electrolytes, BMS optimization, and recycling technologies. The environmental adaptability, safety, and economic viability of batteries are key research areas, and the industry is expected to undergo more innovation and transformation.

We specialize in battery preparation technology research, focusing on overcoming existing energy storage challenges by innovating in electrode materials, battery chemistry, and manufacturing processes to improve performance, enhance safety, and reduce costs. Sustainability and recycling technologies for batteries are also emphasized to mitigate environmental impacts and foster the growth of green energy.