"C-rate" is a term used to describe the rate at which a battery is charged and discharged. It is a relative quantity used to measure how long a battery can be fully charged or discharged. The definition of the multiplier is based on the rated capacity of the battery.
Charge and Discharge Multiplier C = Charge and Discharge Current (A) / Rated Battery Capacity (Ah).
Battery multiplier performance is the ability of a battery to perform at different charge and discharge rates. It is directly related to the maximum current density that the battery can withstand, and is an important indicator of the battery's ability to charge and
discharge quickly.
The relationship between multiplier and time:
1C discharge means the battery can be discharged within 1 hour.
2C discharge means the battery can be discharged in half an hour.
And so on.
Rate performance plays an important role in evaluating the performance of a battery material or cell, especially in applications that require high charge/discharge rates or high power output.
1. electrode material properties: including conductivity and ion diffusion rate, determining the battery charging and discharging speed.
2. Battery structure design: affects the flow of lithium ions and heat distribution, including electrode thickness and porosity.
3. electrolyte performance: ion conductivity and chemical stability have a direct impact on the charge and discharge rate.
4. Battery Temperature: Appropriate temperature range helps to optimize the charge/discharge rate and cycle stability of the battery.
5. Battery internal resistance: lower internal resistance helps to improve the performance of the battery under high rate conditions.
6. Battery aging: As the usage time increases, the battery performance will decrease due to aging, which affects the multiplier performance.
7. Charging strategy and BMS: Reasonable charging strategy and effective battery management system are critical to maintain battery performance.
Together, these factors determine the performance of the battery under high-multiplier charging and discharging conditions.
1. Power Density: High multiplicity performance indicates that the battery is capable of releasing a large amount of power in a short period of time, which is especially important for applications that require fast charging and discharging (e.g., electric vehicles,
starter batteries, portable electronic devices). High power density improves device responsiveness and performance.
2. Charging speed: high rate performance also means that the battery can be quickly charged in a shorter period of time. For applications that require frequent charging, such as electric vehicles, this characteristic can significantly improve ease of use and efficiency.
3. Cycle life: Under high rate discharge conditions, battery materials may experience greater stress, which can affect the cycle life and stability of the battery. Good multiplier performance not only involves high discharge capacity for a short period of time, but also means that the battery can maintain good performance over many charge/discharge cycles.
4. Safety: Battery safety is also a key issue during rapid charge/discharge. Batteries with high rate performance need to be designed with safety in mind to prevent overheating, expansion or other potential hazards.
5. Balance of Energy Density and Power Density: High-rate performance usually comes at the expense of energy density, as the battery needs to be designed to withstand higher discharge rates. In practice, the right balance between energy density (long time energy storage) and power density (short time energy release) needs to be found to meet specific needs.
1. Material Performance Verification: The actual performance of the battery material can be evaluated by determining the performance of the battery at different multiplications (C-rates) in laboratory testing. This helps to verify that the material meets the expected power density requirements, as well as its stability at high discharge rates.
2. Optimization of material formulations: Laboratory testing can help scientists understand the performance of different material formulations under high-rate discharge conditions, thus guiding the optimization and improvement of materials. For example, by testing different electrode materials or additives, the best combination can be found to improve the multiplier performance.
3. Testing battery design: Multiplier performance tests can reveal potential problems in battery design, such as electrode structure, electrical conductivity, and ionic conductivity. These test results help to improve the battery design to enhance its performance in real-world applications.
4. Determine the scope of application: Through laboratory testing, it is possible to determine the scope of application of battery materials or batteries in different application scenarios. For example, certain batteries may perform well at high rates but not be efficient enough under regular use conditions. Laboratory testing can help identify these differences in suitability.
5. Predicting Long-Term Performance: High-rate testing in the lab can help predict the long-term performance of a battery in actual use. This includes analyzing the effect of high rate discharges on battery life and helping to predict battery decline patterns under high load conditions.
6. Standardized testing: Laboratory testing provides a standardized method of evaluating different battery materials and battery systems, ensuring comparability between different products. This standardized testing helps researchers and manufacturers compare different battery technologies and make more informed choices.
1. suitable H2O-solute system is crucial for designing antifreeze electrolytes for low-temperature aqueous batteries (LTABs). Yongsheng Hu and Yaxiang Lu from the Institute of Physics, Chinese Academy of Sciences (CAS), Yijun Lu from the Chinese University of Hong Kong (CUHK), and Junmei Zhao from the Institute of Process Engineering, CAS, have proposed a general strategy to create a polysolvent system by introducing either auxiliary salts with high ionic potential cations (e.g., Al3+, Ca2+) or co-solvents with high donor numbers (e.g., ethylene glycol) to achieve an electrolyte with low Te and strong SCA .
To demonstrate the battery performance at different temperatures using the designed electrolytes, the authors synthesized the Prussian blue-like anode NaFeMnHCF (Na1.65Fe0.21Mn0.79[Fe(CN)6]0.92-2.08H2O) and prepared two anode materials: the polyanionic anode NaTi2(PO4)3, which was used to achieve high energy density, and the ultra-low-temperature operation of organic anode 3,4,9,10-perylenetetracarboxylic acid diimide (PTCDI). At 25°C, the NaFeMnHCF/Na-H2O-EG/NaTi2(PO4)3 full cell provided an energy density of 80 Wh kg-1 at 2C and maintained 70% capacity retention after 5000 cycles at 8C. At -80°C, the NaFeMnHCF/Na-H2O-Ca/PTCDI button cell provides a high energy density of 20 Wh kg-1 and maintains 93.1% capacity retention after 20 cycles at 0.1C. In addition, even at -85°C, the button cell provides an energy density of 12.5 Wh kg-1 at 0.1C.
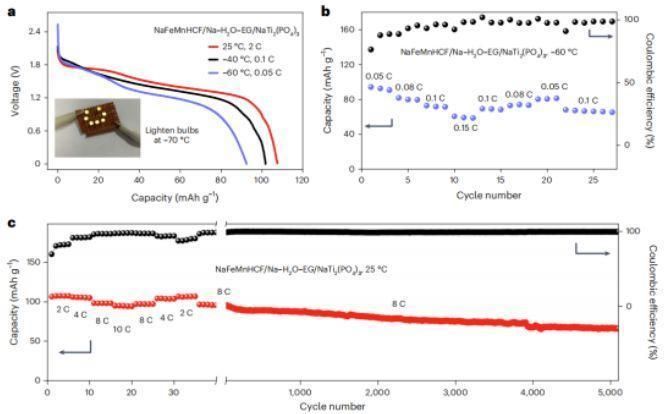
Fig. 1 Electrochemical performance graph of aqueous battery
2. All-solid-state sodium batteries (ASSSBs) are large-scale, safe, low-cost energy storage devices that can replace lithium batteries. Professor Linda F. Nazar of the University of Waterloo, Canada, published a research article entitled “4V Na Solid State Batteries Enabled by a Scalable Sodium Metal Oxyhalide Solid Electrolyte” in ACS Energy Letter. NaTaOCl4, a Na+ fast ion conductor, was prepared by a mild mechanical reaction synthesis method.
P2-type Na2/3Ni1/3Mn2/3O2 (NNMO) was chosen as the high-voltage positive electrode to demonstrate the electrochemical properties of NTOC. Na3Sn/Na2Sn was used on the negative side and a thin layer of Na4(CB11H12)2(B12H12) was used to protect the negative electrode from the reduction of Na3Sn/Na2Sn. ASSSB also exhibited excellent multiplicity performance using Na2Sn instead of Na3Sn as the anode material for rate testing (Fig. c). The multiplicity performance was tested in this work at multiplicities of 0.1C, 0.2C, 0.5C, 1C, and 0.1C, with 5 cycles at each rate.
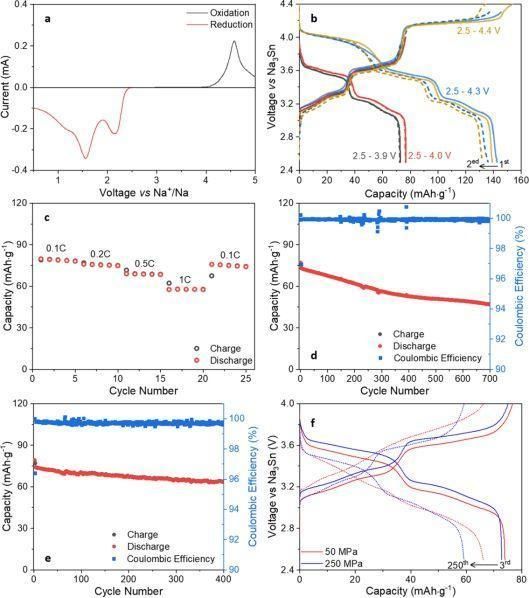
Fig. 2 Electrochemical performance graph of solid-state battery
When setting the constant current charge/discharge work step, you can directly input the current value. You can also select the multiplier mode at the bottom of the page, input the nominal specific capacity and mass of active material, and then input the multiplier value, the software can automatically calculate the current value under the corresponding multiplier. The multiplier mode can be used to set the time of the step where the current value needs to be entered.
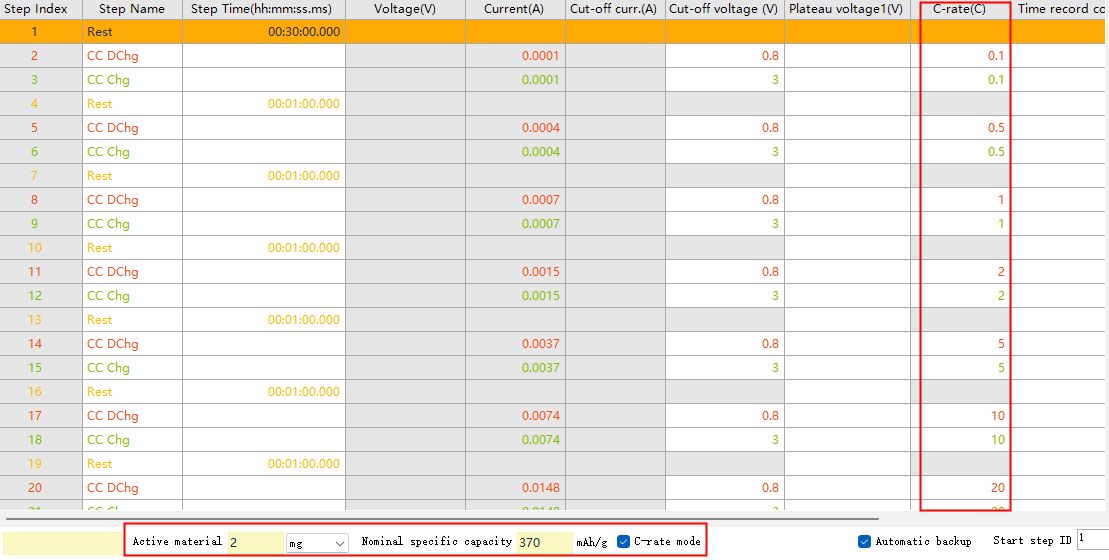
Fig. 3 BTS software multiplier mode introduction diagram
If there is no multiplier option on the work step setting page, go back to the top of the main page, click “Settings”, select “System Settings”, and then click “Work Step Edit” on the left side of the page.
Under “Step Parameter Settings”, select “Multiplier” on the right side. Click on the arrow symbol in the center to change the “Magnification” status from hidden to shown.
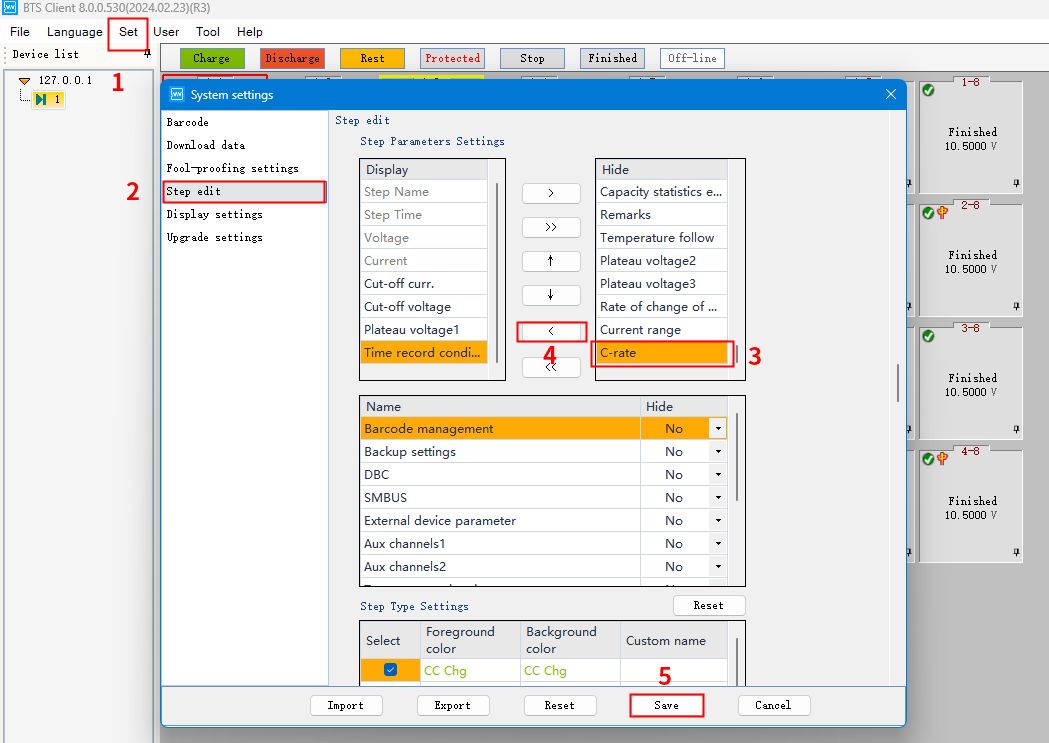
Fig. 4 Calling up the BTS software C-rate function
Continue to scroll down the page, you can choose to set the multiplier step parameter as “Nominal capacity” or “Nominal specific capacity”, according to the needs of the settings can be.
Then click save, enter user name: admin, password: neware, click confirm.
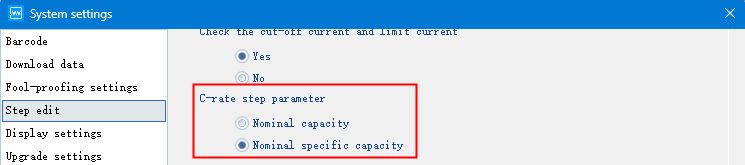
Fig. 5 C-rate step parameter setting
Go back to the main page of the channel, right click and select “Unit settings”, you can modify the specific volume unit, there are several units to choose.
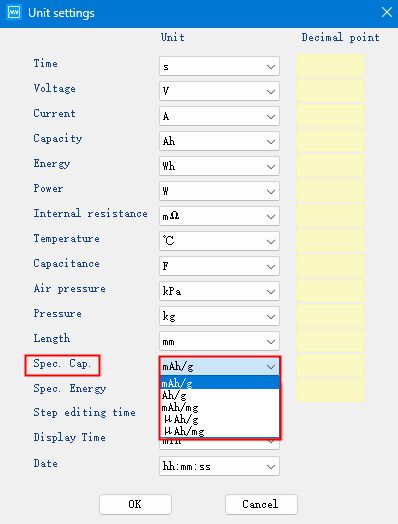
Fig. 6 Modifying the specific capacity unit
Take the study of negative electrode material as an example, first set up constant current discharge work step, then set up constant current charging work step. Check the “C-rate Mode” at the bottom of the page, input different multiplication parameters, and automatically calculate the current value corresponding to the rate. The common rate gradient is 0.1C, 0.5C, 1C, 2C, 5C, 10C, 20C.
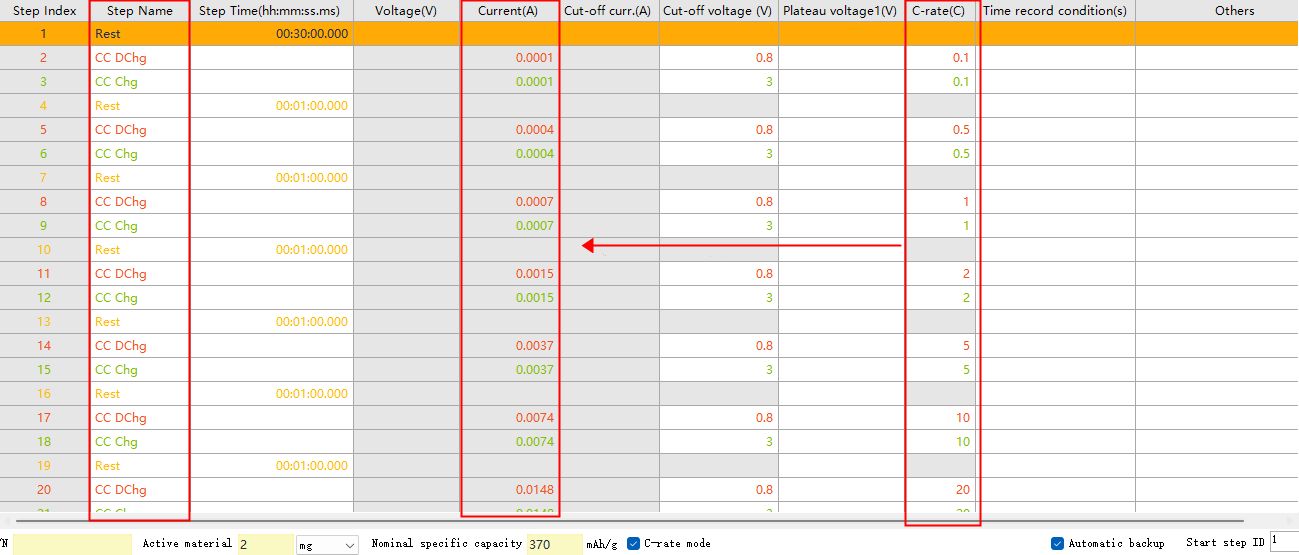
Fig. 7 BTS8.0 software rate step setting interface
You can set the recording conditions and protection conditions by double-clicking the blank cell under “Others” on the right side.
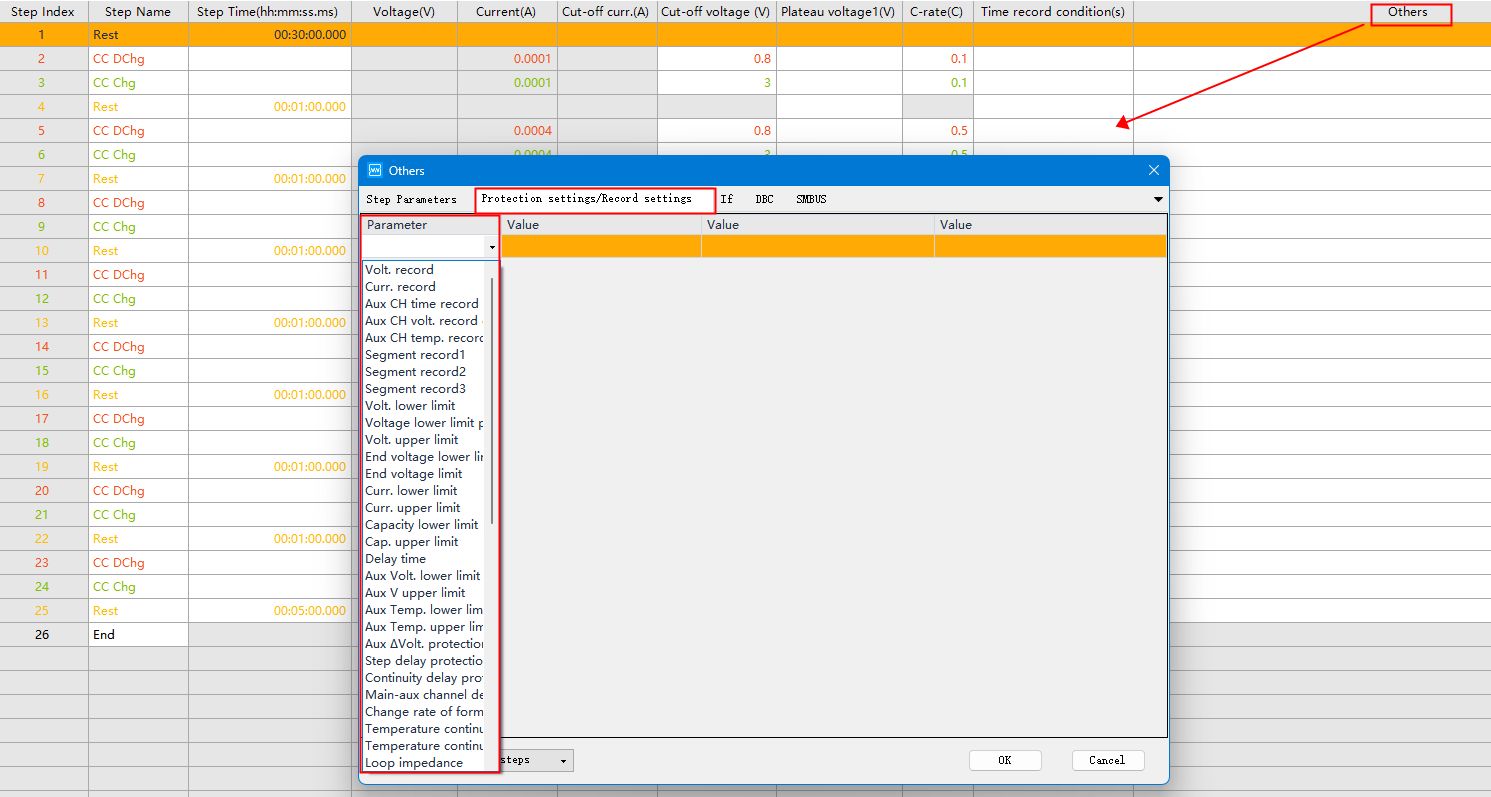
Fig.8 Setting up recording conditions and protection conditions in BTS software
Open the BTSDA software, right click on the top of the graph area and select “Curve Settings”.
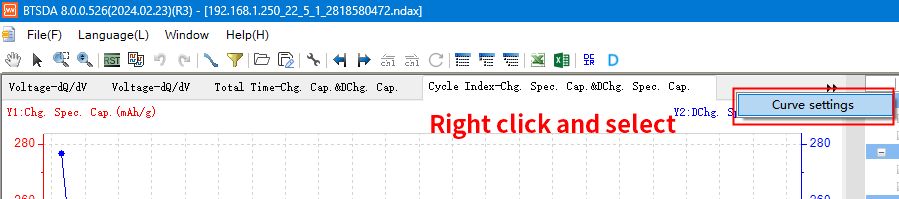
Fig. 9 Curve setting function of BTSDA software
Set X-axis as cycle number, Y1-axis as charging specific capacity, Y2-axis as discharging specific capacity, and then click OK to display the multiplier performance graph.
Note: This is only for operation demonstration, you can modify the axis parameters according to your needs.
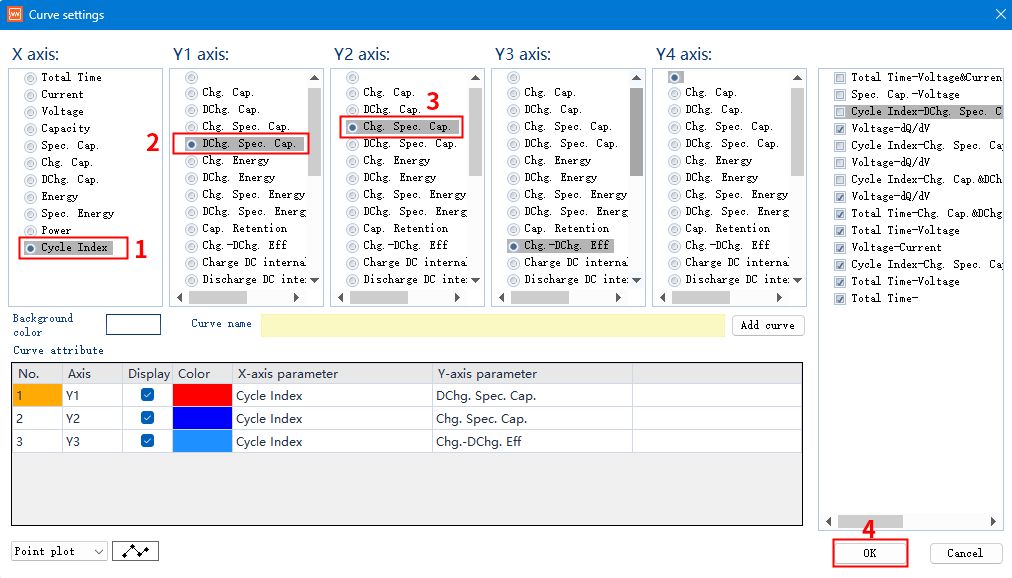
Fig. 10 Setting curve parameters
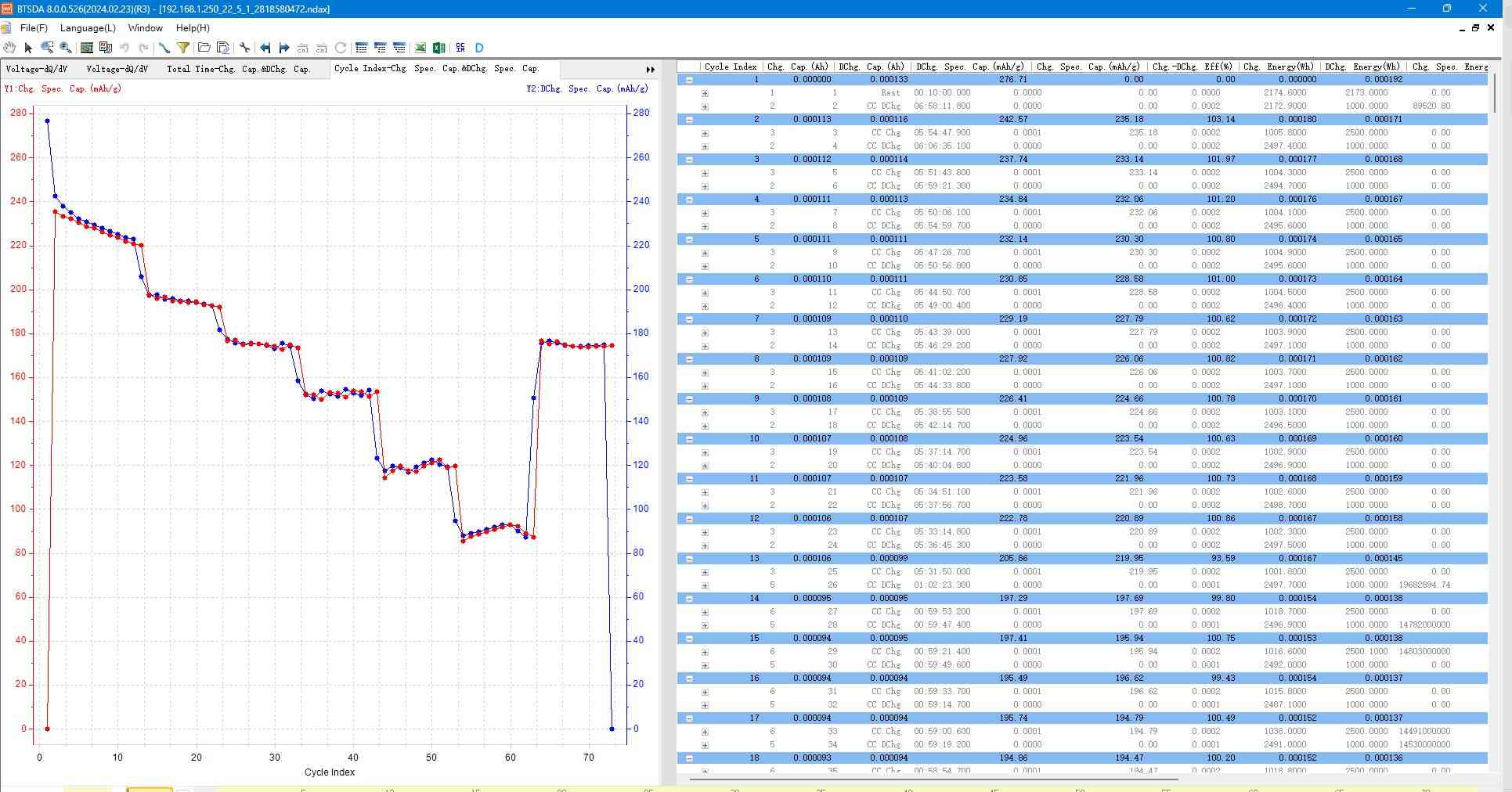
Fig. 11 Rate performance graph
Right click on the chart area to select items such as “Copy graph” & “Copy data”.
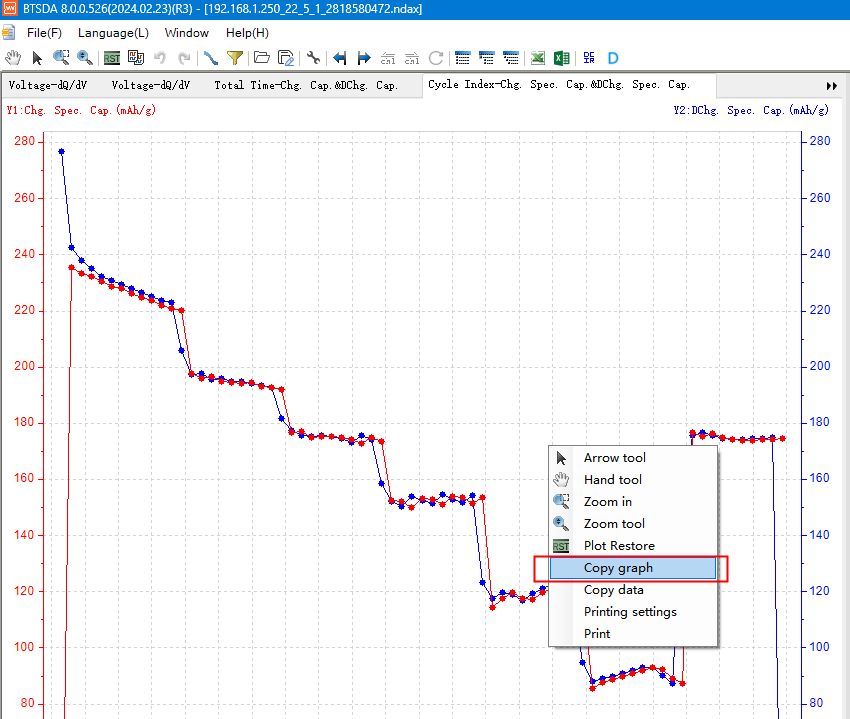
Fig. 12 Copying graphical function interface
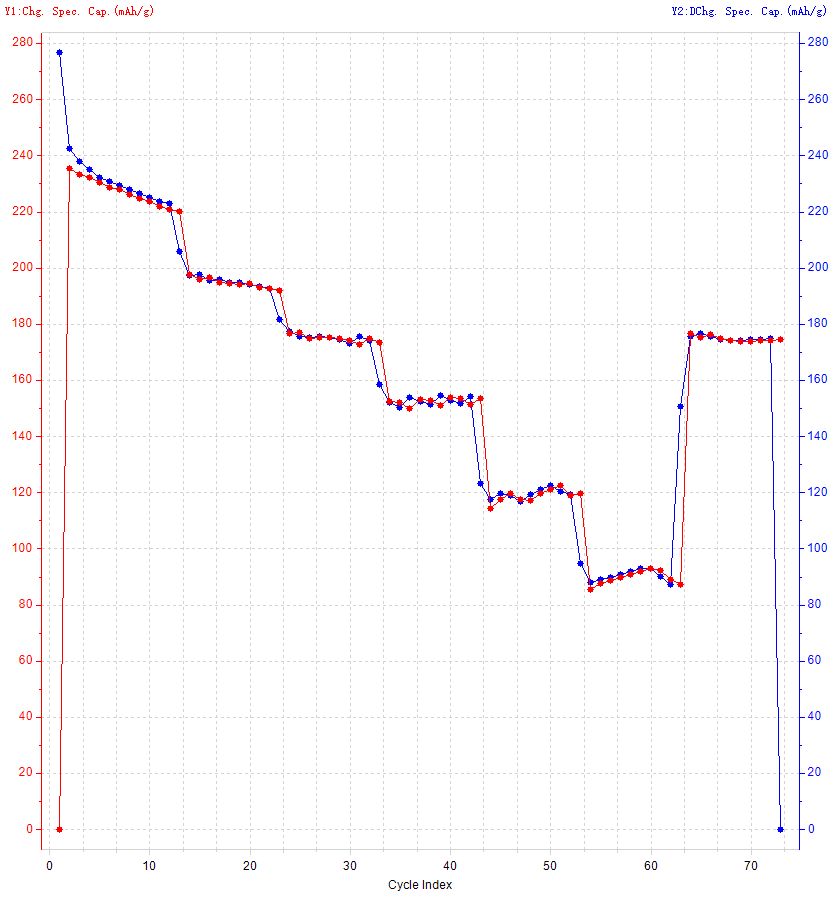
Fig. 13 Graph of data copied from BTSDA
Double-click the axes to adjust the graph by modifying the axes parameter unit, range, title, primary scale size, secondary scale size and other parameters.
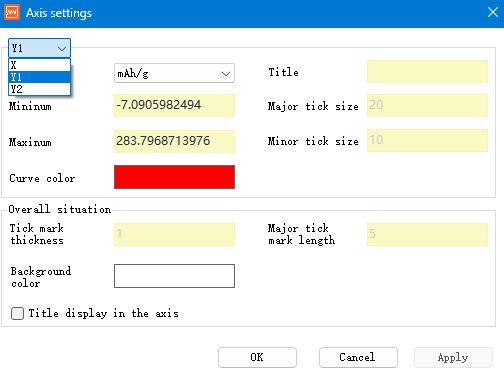
Fig. 14 Axis settings interface
The data displayed in the chart can be copied directly to an Excle table. It is more convenient than exporting reports.
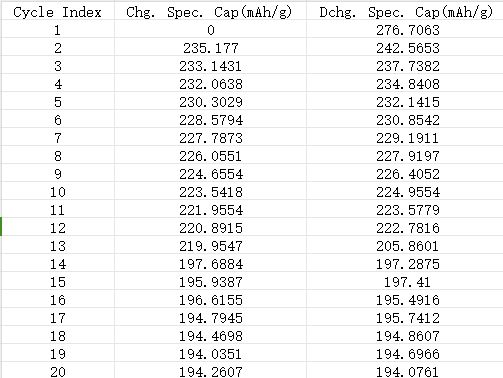
Fig. 15 Partial display of data exported
More BTS software operation videos can be viewed in the Support-BTS Tutorial section of the neware.net website.




Technology
December 04, 2025

Technology
November 30, 2025

The lab focuses on solid-state battery research to overcome traditional lithium batteries' safety and energy density issues, supporting environmental sustainability. It develops innovative solid-state electrolytes, refines electrode materials, and investigates ion transfer and interface stability to revolutionize battery technology.

The electric vehicle battery industry is rapidly developing, focusing on technological innovation, market competition, and sustainability. Research hotspots include solid-state batteries, new types of electrolytes, BMS optimization, and recycling technologies. The environmental adaptability, safety, and economic viability of batteries are key research areas, and the industry is expected to undergo more innovation and transformation.

We specialize in battery preparation technology research, focusing on overcoming existing energy storage challenges by innovating in electrode materials, battery chemistry, and manufacturing processes to improve performance, enhance safety, and reduce costs. Sustainability and recycling technologies for batteries are also emphasized to mitigate environmental impacts and foster the growth of green energy.