Primary battery
A device that spontaneously sends an electric current to an external circuit load connected with two electrodes and the external circuit
A primary battery, also known as a non-rechargeable battery or a dry cell, is a device that spontaneously generates an electric current and delivers it to an external circuit load connected to its two electrodes. It typically consists of one or more chemical substances that undergo a chemical reaction to produce electrical energy.
The operation of a primary battery relies on electrochemical processes occurring during the chemical reaction. The battery has two electrodes: an anode and a cathode . These electrodes are separated by an electrolyte, which can be a liquid or a solid.
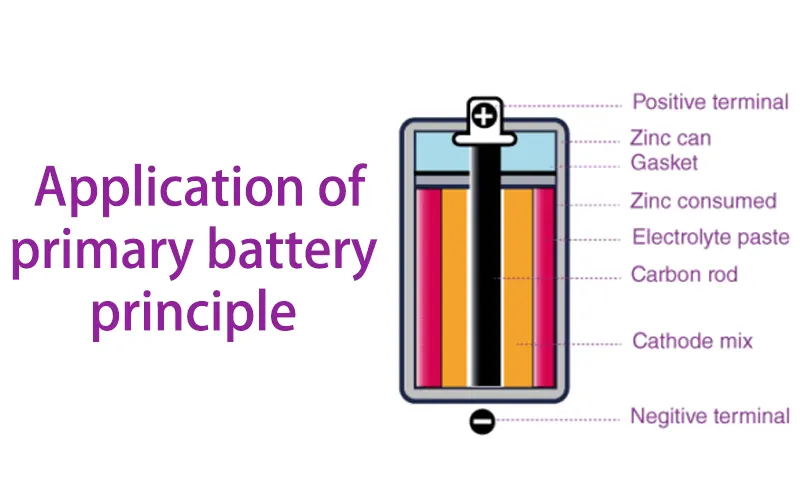
When an external circuit is connected to the battery's electrodes, the chemical reaction begins. The anode undergoes an oxidation reaction, while the cathode undergoes a reduction reaction. These reactions cause electrons to flow between the electrodes, creating an electric current. This current can then power devices or loads connected to the external circuit.
One important characteristic of primary batteries is that they are non-rechargeable. Once the chemical reaction inside the battery is depleted or reaches an irreversible state, the battery cannot be recharged or reused. It needs to be replaced with a new battery to restore its functionality.
Positive electrode/Cathode
The electrode that occurs reduction reaction with higher voltage
The positive electrode, also known as the cathode, is an essential component in a battery. In electrochemistry, the positive electrode is the electrode where reduction reactions occur, typically at a higher potential or voltage. It serves as the positive polarity plate in a battery, attracting the flow of electrons from the negative electrode (anode) towards the positive electrode. The positive electrode usually consists of active materials or reactants.
In a typical battery, the positive electrode is separated from the negative electrode by an electrolyte, forming the electrochemical system of the battery. When an external circuit is connected to the positive and negative electrodes of the battery, the positive electrode undergoes a reduction reaction. In the reduction reaction, the positive electrode accepts electrons and reacts with ions or chemical species to maintain the charge balance of the battery.
The selection of the positive electrode plays a crucial role in battery design and performance. Different types of batteries use different positive electrode materials, such as carbon rods in zinc-carbon batteries, lead dioxide and lead-calcium alloys in lead-acid batteries, and lithium compounds like lithium cobalt oxide, lithium iron phosphate, etc., in lithium-ion batteries. The choice of positive electrode material determines the battery's voltage, capacity, cycle life, and other characteristics.
In summary, the positive electrode is the electrode in a battery where reduction reactions occur at a higher voltage. It serves as the counterpart to the negative electrode, acting as the positive polarity plate, attracting the flow of electrons from the negative electrode to maintain charge balance. The selection of positive electrode materials significantly influences the battery's performance and characteristics.
Negative electrode/Anode
The negative electrode is the electrode in a battery that undergoes an oxidation reaction at a lower voltage
The negative electrode, also known as the anode, is an essential component in a battery. In electrochemistry, the negative electrode is the electrode where oxidation reactions occur, typically at a lower potential or voltage. It serves as the negative polarity plate in a battery, providing a pathway for the flow of electrons from the positive electrode (cathode) to the negative electrode. The negative electrode usually consists of active materials or reactants.
In a typical battery, the negative electrode is separated from the positive electrode by an electrolyte, forming the electrochemical system of the battery. When an external circuit is connected to the negative and positive electrodes of the battery, the negative electrode undergoes an oxidation reaction. In the oxidation reaction, the negative electrode loses electrons and reacts with ions or chemical species to maintain the charge balance of the battery.
The selection of the negative electrode plays a crucial role in battery design and performance. Different types of batteries use different negative electrode materials, such as zinc in zinc-carbon batteries, lead in lead-acid batteries, graphite in lithium-ion batteries, and so on. The choice of negative electrode material determines the battery's voltage, capacity, cycle life, and other characteristics.
In summary, the negative electrode is the electrode in a battery where oxidation reactions occur at a lower voltage. It serves as the counterpart to the positive electrode, acting as the negative polarity plate, providing a pathway for the flow of electrons from the positive electrode to maintain charge balance. The selection of negative electrode materials significantly influences the battery's performance and characteristics.
Electrode
The electronic conductor or semiconductor in contact with an electrolyte solution or electrolyte
An electrode is an electronic conductor or semiconductor in direct contact with an electrolyte solution or electrolyte. Electrodes have wide applications in the field of electrochemistry, including energy storage devices, electrochemical sensors, electrolysis cells, and more. Let's provide a detailed explanation of electrodes from a professional perspective and provide some practical examples.
Electrodes are crucial components in electrochemical reactions as they provide pathways for the input and output of electric current. In an electrolysis cell, electrodes are divided into anode and cathode. The anode is where the current enters the electrolyte from the external circuit, while the cathode is where the current enters the external circuit from the electrolyte.
In batteries, the negative electrode (cathode) is typically composed of active materials such as zinc in zinc-carbon batteries or graphite in lithium-ion batteries. The positive electrode (anode) consists of reactant materials such as carbon in zinc-carbon batteries or lithium cobalt oxide in lithium-ion batteries. These electrode materials undergo oxidation-reduction reactions during charge and discharge processes, releasing or absorbing electrons.
Another example is the working electrode in electrochemical sensors. Common materials for working electrodes include gold, platinum, carbon nanotubes, and more. These electrodematerials exhibit good electrical conductivity and chemical stability, making them suitable for detecting chemical concentrations and biological molecule activities in the environment.
Furthermore, electrodes are also used in electrolytic cells for electroplating or electrolysis processes. In electrolysis, the selection of electrode materials is crucial as they need to withstand conditions such as high temperature, corrosive substances, and current density. For example, the cathode in an aluminum electrolytic cell is typically made of carbon material, while the anode is made of conductive iron.
The design and performance of electrodes are critical for the effectiveness of electrochemical applications. The selection of electrode materials should consider factors such as electrical conductivity, chemical stability, and reaction activity. Additionally, the morphology and structure of the electrode surface also play an important role in the rate and efficiency of electrochemical reactions.
Reference electrode
An electrode close to the ideal non-polarization with a known potential and no current, which is used to test voltage of woork electrdod
A reference electrode is an electrode that closely approximates the ideal non-polarization state, with a known and stable potential and no current output. It is used as a standard reference point for measuring the voltage of a working electrode. Let's provide a detailed explanation of reference electrodes from a professional perspective and provide some practical examples.
Reference electrodes play a crucial role in the field of electrochemistry as they serve as a reference for potential measurements. Compared to the working electrode, a reference electrode should have a stable and known potential that is not affected by electrochemical reactions. This allows the reference electrode to provide a reliable reference potential for measuring the potential difference of the working electrode.
One common example of a reference electrode is the Standard Hydrogen Electrode (SHE). The potential of the Standard Hydrogen Electrode is defined as zero and is based on the potential of hydrogen gas in contact with an acidic solution at 1 atm pressure. The potential of the Standard Hydrogen Electrode is constant and serves as a reference potential for measuring the potential of other electrodes.
In addition to the Standard Hydrogen Electrode, there are other types of reference electrodes such as the Saturated Calomel Electrode (SCE) and the Silver/Silver Chloride Electrode (Ag/AgCl). These referenceelectrodes also have known potentials and provide stable reference potentials.
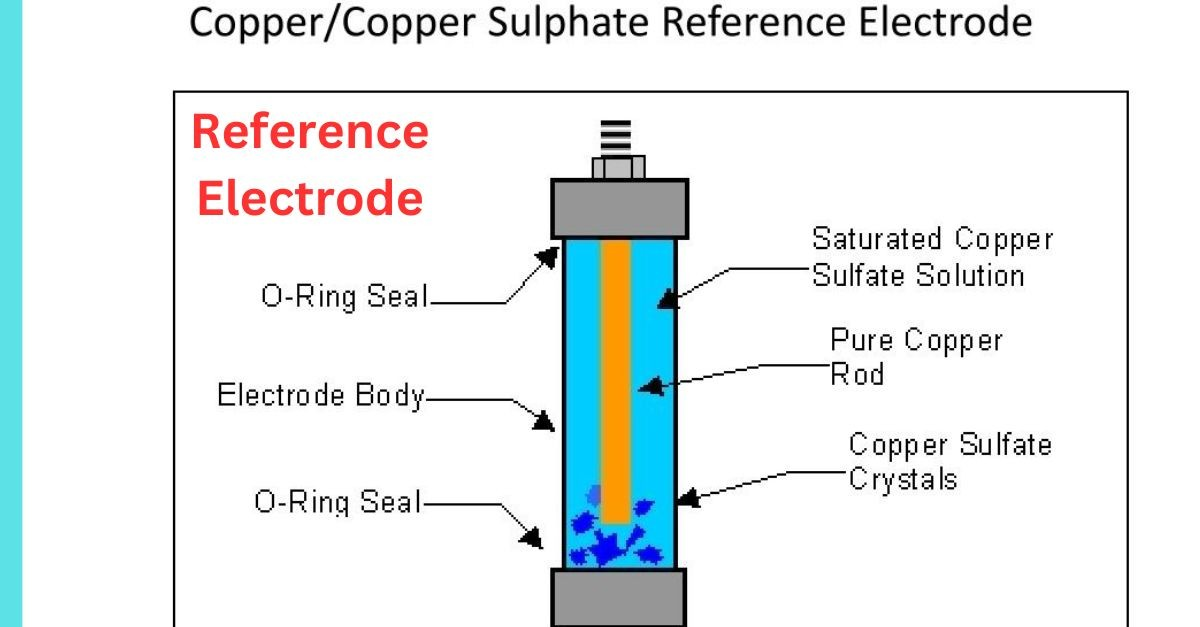
In practical applications, reference electrodes are typically used in conjunction with a working electrode to measure the potential difference in an electrochemical system. By connecting the reference electrode and the working electrode and immersing them in an electrolyte solution, a voltmeter or potentiometer can be used to measure the potential difference between the two electrodes.
The choice of reference electrode depends on the specific application requirements. In some applications, reference electrodes with a higher potential range may be required, such as for studying high-potential electrochemical reactions or battery testing. In such cases, a platinum electrode or other high-potential reference electrode may be more suitable.
In summary, a reference electrode is an electrode that closely approximates the ideal non-polarization state, with a known and stable potential and no current output. It serves as a standard reference point for potential measurements in electrochemical applications and provides a reliable reference potential. By using it in conjunction with a working electrode, a reference electrode can be used to measure the potential difference in electrochemical systems and plays an important role in research, analysis, and industrial applications.











