Binders play a key role in a variety of industrial and scientific applications, but their failure problems often lead to serious performance degradation and economic losses. Therefore, it is important to understand and analyze the causes of binder failure and adopt appropriate testing and characterization tools to safeguard their performance and extend their service life. In this paper, we will discuss in detail the basic principle of failure mode of binder and its test and characterization means.
Binder is a substance that can firmly combine two or more materials together. According to its composition and application areas of different adhesives can be divided into organic adhesives, inorganic adhesives, natural adhesives and synthetic adhesives. Different types of each type of binder have their unique advantages in terms of their properties and fields of application.
The action mechanism of adhesives mainly includes physical action and chemical action. Physical action includes mechanical embedding, electrostatic attraction, van der Waals force, etc., while chemical action includes chemical binder, hydrogen bonding, ionic bonding, etc. In practical application, adhesives can be used in a variety of ways. In practice, the adhesive bonding performance of adhesives is usually the result of a variety of action mechanisms.
Factors affecting the performance of adhesives include chemical composition, physical structure, environmental conditions and conditions of use of adhesives, etc. The performance of different adhesives in different application environments will be different. Therefore, choosing the right binder and optimizing its use conditions are crucial for improving its bonding performance.
Adhesive failure refers to the deterioration of adhesive performance or loss of adhesive ability due to various reasons during the use of adhesives, thus failing to meet their expected functional performance. Adhesive failure not only affects the quality and performance of the product, but also may lead to safety hazards and economic losses.
1. Contact interface damage: One of the main mechanisms of adhesive failure is contact interface breakdown, i.e., the loss of adhesion between the adhesive and the object to be adhered. This is usually due to insufficient interfacial interaction force of the binder to effectively maintain the bond between the electrode material and the fluid collector. This is manifested by the gradual detachment of the adhesive layer during the electrode cycling process, which leads to the separation of the active material from the electrode surface, thus hindering the ion and electron transport paths and ultimately causing the degradation of the battery capacity.
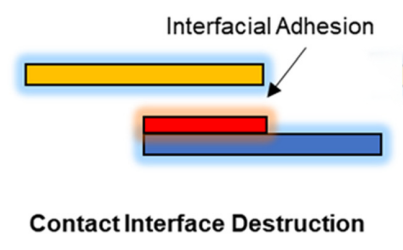
Figure 1 contact interface disruption
In the electrode preparation process, the selection and use of binder directly affects the quality of interfacial bonding. Generally, the binder needs to form a stable interface between the electrode material and the fluid collector to ensure the smooth transport of electrons and ions. However, since electrodes undergo repeated charging and discharging during cycling, the degradation of interfacial bonding is a common problem. Especially under high rate charging and discharging conditions, the volume change of the electrode material leads to stress concentration at the interface, which accelerates the destruction and detachment of the interface.
2. Binder fracture: Binder fracture is another common form of failure. The binder material undergoes changes in stress and strain during electrode cycling, and if the mechanical strength of the binder is insufficient, fracture is likely to occur. Especially under high rate charging and discharging and high current conditions, the expansion and contraction of the binder material will exacerbate its tendency to fracture. This not only affects the structural integrity of the electrode, but also may trigger the pulverization and dispersion of the electrode material, further degrading the battery performance.
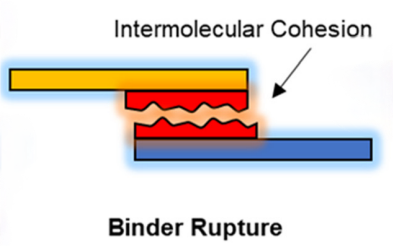
Figure 2 Binder rupture
To avoid binder fracture, researchers have attempted to enhance the mechanical strength of the binder by introducing cross-linked structures and high molecular weight polymers. For example, by utilizing copolymerization and cross-linking techniques, physical and chemical cross-linking points can be formed between the molecular chains of the binder, thereby improving its fracture toughness and fatigue resistance. In addition, by adjusting the molecular weight and cross-linking density of the binder, its mechanical properties can be further optimized so that it remains stable during electrode cycling.
3. Destruction of the adherend: Destruction of the binder itself is also a cause of binder failure. Although the binder maintains a good adhesion and mechanical strength, if structural damage occurs to the binder (e.g., the active material or the collector) during the electrode cycling process, it can also lead to electrode failure. For example, the active material particles of highly loaded electrodes may undergo cracking and detachment after many cycles, leading to a sharp drop in battery capacity.
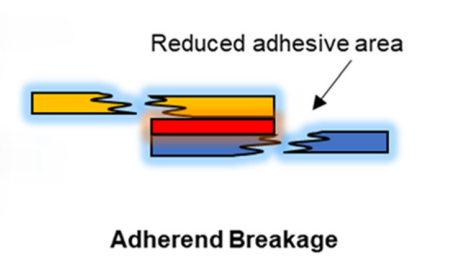
Figure 3 Binder rupture
In practice, the selection and design of electrode materials also directly affects the failure mechanism of the binder. For high specific energy batteries, electrode materials usually have high energy density and high activity, but this also means that their structures are more susceptible to cyclic stress. Therefore, the development of electrode materials with high mechanical strength and stability, as well as the binder that matches them, is the key to improving battery performance and lifetime.
The causes of binder failure include chemical factors, physical factors and mechanical factors, etc.
1. chemical factors: chemical factors include the chemical reaction between the binder and the bonded material, the chemical degradation of the binder and the erosion of the binder by the chemicals in the environment.
2. physical factors: physical factors include temperature, humidity, light and radiation and other environmental conditions on the binder, these factors may lead to changes in the physical properties of the adhesive, thus affecting its bonding properties
3. Mechanical factors: Mechanical factors include external stress concentration and fatigue, etc. These factors may lead to a decrease in the mechanical properties of the adhesive, thus affecting its bonding performance.
Physical test methods mainly include microscope observation, scanning electron microscope (SEM) and X-ray diffraction (XRD), etc.
1. microscope observation: microscope observation is one of the most basic physical test methods, through the optical microscope can observe the surface morphology and microstructure of the binder after failure, so as to make a preliminary judgment of the failure mode and reasons.
2. Scanning electron microscope (SEM): Scanning Electron Microscope (SEM) is a kind of high resolution microscope. Through SEM, the microstructure of the binder after failure can be observed in detail, and the surface morphology, fracture pattern and interfacial bonding of the binder after failure can be obtained.
3. X-ray diffraction (XRD): X-ray diffraction (XRD) is a commonly used test method to analyze the crystal structure of materials. Through XRD, the crystal structure and phase composition of the binder after failure can be determined to analyze the cause of binder failure.
Chemical testing methods mainly include infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS) and energy dispersive X-ray spectroscopy (EDS), etc.
1. Infrared spectroscopy (FTIR): Infrared spectroscopy (FTIR) is a commonly used test method to analyze the chemical structure of materials, through the FTIR can determine the chemical bonding of the binder failure and functional group changes to analyze the reasons for the failure of the binder.
2. X-ray photoelectron spectroscopy (XPS): X-ray Photoelectron Spectroscopy (XPS) is a commonly used test method to analyze the chemical composition of the surface of the material, through the XPS can determine the chemical composition of the surface after the failure of the binder and the valence distribution to analyze the cause of the failure of the binder.
3. Energy dispersive X-ray spectroscopy (EDS): Energy dispersive X-ray spectroscopy (EDS) is a commonly used test method to analyze the elemental composition of materials, through the EDS can determine the elemental composition and distribution of the failure of the binder to analyze the reasons for the failure of the binder.
Mechanical test methods mainly include tensile test, shear test and fatigue test:
1.tensile test: tensile test is a commonly used method to test the mechanical properties of materials through the tensile test can be determined before and after the failure of the tensile strength and elongation of the binder to analyze the reasons for the failure of the binder.
2. shear test: shear test is a commonly used method to test the shear strength of the binder through the shear test can be determined before and after the failure of the binder shear strength to analyze the causes of failure of the binder.
3. fatigue test: fatigue test is a commonly used method to test the fatigue properties of materials through the fatigue test can determine the fatigue life of the binder under cyclic loading and the failure mode to analyze the causes of failure of the binder.
Comprehensive testing methods mainly include photoelectron spectroscopy (PES), thermal analysis (DSC/TGA) and dynamic mechanical analysis (DMA):
1. Photoelectron spectroscopy (PES): photoelectron spectroscopy (PES) is a commonly used test method to analyze the electronic structure of the surface of the material through the PES can determine the electronic structure of the surface of the binder after the failure of the distribution of energy bands, so as to analyze the reasons for the failure of the binder.
2. Thermal analysis (DSC/TGA): Thermal Analysis (DSC/TGA) is a commonly used test method to analyze the thermal properties of materials through the DSC can determine the glass transition temperature (Tg) and melting point of the binder through the TGA can be determined by the binder's thermal stability and decomposition temperature in order to analyze the causes of binder failure.
3. Dynamic mechanical analysis (DMA): Dynamic Mechanical Analysis (DMA) is a commonly used method to test the dynamic mechanical properties of materials through the DMA can be determined by the energy storage modulus (E') and loss modulus (E'') of the binder, so as to analyze the causes of failure of the binder.
To address the above failure mechanisms, researchers have proposed a variety of modification methods to improve the performance of binder, mainly including molecular design and functionalization modification.
Molecular design is a method to enhance the interfacial interaction force and mechanical strength of a binder by changing its molecular structure. Common design ideas include the introduction of functional groups with stronger interfacial interactions, such as hydrogen bonding, Coulombic attraction and π-π stacking, to replace the weak van der Waals forces. Dopamine analogs inspired by mussels are widely used in the design of binders due to their strong adhesion. For example, by introducing catechol functional groups into binder molecules, their adhesion and mechanical strength can be significantly improved.
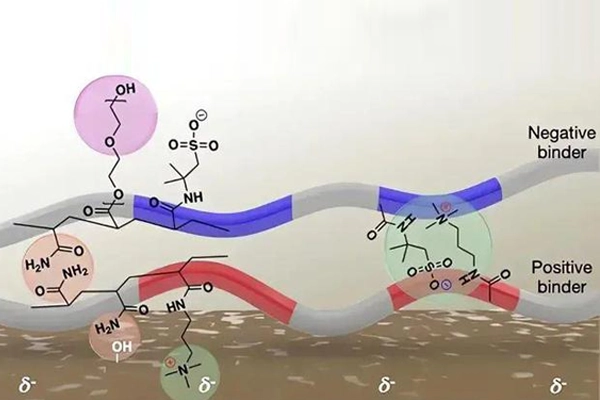
Figure 4 Molecular structure design
Through molecular design, the molecular weight and molecular structure of the binder can also be adjusted to achieve specific performance needs. For example, utilizing high molecular weight polymers can improve the mechanical strength and thermal stability of the binder, while low molecular weight polymers can improve its flexibility and adhesion properties. In addition, through copolymerization and grafting techniques, different functional groups can be introduced into the binder molecule for multifunctionality and customized performance optimization.
Functionalization modification is a way to improve the performance of binder through chemical modification methods, and the commonly used modification methods include grafting, copolymerization, cross-linking and blending. By introducing functional groups with different molecular structures and interaction forces into the binder, customized performance optimization can be achieved. For example, grafting catechols into alginate (Alg-C) and polyacrylic acid (PAA-C) can significantly enhance adhesion and mechanical strength. In addition, the mechanical properties and electrochemical stability of the binder can be further optimized by modulating its molecular weight and crosslink density.
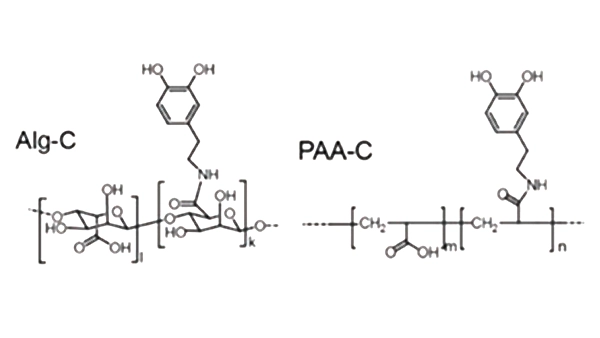
Figure 5 Binder functionalization modification
Functionalization modification can also be achieved by surface modification. For example, by introducing hydrophilic or hydrophobic functional groups on the surface of the binder, its interaction with the electrode material and the electrolyte can be regulated, thereby improving its interfacial stability and electrochemical properties. In addition, reinforcement using nanoparticles and nanofibers is an effective way to improve the performance of the binder. These nanomaterials can form a uniformly dispersed reinforcing phase in the binder and improve its mechanical strength and wear resistance.
Adhesive failure is the result of a complex multifactorial integrated effect, through the in-depth analysis of the failure mode and causes of adhesives and the adoption of appropriate means of testing and characterization can be effective in identifying and solving the problem of adhesive failure, and to improve the adhesive bonding performance and service life of adhesives. In practical application, rational selection of binder, optimization of bonding process and strengthening of quality control are effective measures to prevent adhesive failure. Through continuous improvement and innovation of binder technology, the value and prospect of its application in various fields can be further enhanced.




The lab focuses on solid-state battery research to overcome traditional lithium batteries' safety and energy density issues, supporting environmental sustainability. It develops innovative solid-state electrolytes, refines electrode materials, and investigates ion transfer and interface stability to revolutionize battery technology.

The electric vehicle battery industry is rapidly developing, focusing on technological innovation, market competition, and sustainability. Research hotspots include solid-state batteries, new types of electrolytes, BMS optimization, and recycling technologies. The environmental adaptability, safety, and economic viability of batteries are key research areas, and the industry is expected to undergo more innovation and transformation.

We specialize in battery preparation technology research, focusing on overcoming existing energy storage challenges by innovating in electrode materials, battery chemistry, and manufacturing processes to improve performance, enhance safety, and reduce costs. Sustainability and recycling technologies for batteries are also emphasized to mitigate environmental impacts and foster the growth of green energy.