A liquid rechargeable Lithium-ion battery, essentially, is a type of battery that incorporates only liquid electrolytes in its electrochemical system. This battery design relies on the movement of ions dissolved in the liquid electrolyte to facilitate the charging and discharging process. Unlike other Lithium-ion batteries that may utilize solid electrolytes or polymer gel electrolytes, the liquid electrolyte within this battery allows for greater ionic conductivity, thereby enhancing the battery's overall performance.
Positive electrode (Cathode): Typically made of a lithium-containing compound such as Lithium Cobalt Oxide (LiCoO2), Lithium Iron Phosphate (LiFePO4), or Lithium Nickel Cobalt Aluminum Oxide (NCA).
Negative electrode (Anode): Commonly composed of carbon-based materials that exhibit a layered structure with numerous micropores, facilitating the intercalation and deintercalation of lithium ions.
The Liquid Electrolyte in Lithium-ion batteries commonly employs several materials that are crucial for its functioning. These materials are typically composed of organic solvents and lithium salts, which facilitate the transport of lithium ions between the electrodes. Here are some common materials used in the Liquid Electrolyte of Lithium-ion batteries.
Ethylene carbonate (EC): A cyclic carbonate commonly used as a solvent in the electrolyte. It provides good stability and high ionic conductivity.
Ethyl methyl carbonate (EMC): Another solvent that is commonly mixed with EC to form a solvent blend. The blend offers a good balance of viscosity, conductivity, and stability.
Dimethyl carbonate (DMC): Used as a solvent in some electrolyte formulations, it contributes to the desired physicochemical properties of the electrolyte.
Lithium salts: Lithium Hexafluorophosphate (LiPF6): A commonly used lithium salt in Liquid Electrolytes due to its high ionic conductivity and solubility in the organic solvents mentioned above. However, it is also known to be thermally unstable and susceptible to hydrolysis.
Additives: Various additives are also included in the electrolyte to enhance its performance, such as vinylene carbonate (VC) or fluoroethylene carbonate (FEC), which are known to improve the battery's cycling stability and safety.
In summary, the Liquid Electrolyte in Lithium-ion batteries is a complex mixture of organic solvents, lithium salts, and additives that work together to facilitate the efficient transport of lithium ions between the electrodes. The choice of materials and their proportions are crucial for achieving the desired physicochemical properties of the electrolyte, which ultimately determine the performance of the Lithium-ion battery.
Separator: A porous material placed between the positive and negative electrodes to prevent direct contact while allowing the passage of lithium ions.
Charging: During the charging process, lithium ions are released from the positive electrode and migrate through the liquid electrolyte to the negative electrode. These ions are then intercalated into the micropores of the carbon-based anode.
Discharging: When the battery is in use, the lithium ions are deintercalated from the negative electrode and migrate back to the positive electrode through the liquid electrolyte. This movement of ions generates an electric current that powers the device.
High energy density: Compared to traditional batteries, liquid rechargeable Lithium-ion batteries offer higher energy densities, meaning they can store more energy per unit mass or volume.
Long life cycle: These batteries typically have a longer life cycle, with the ability to undergo hundreds of charge-discharge cycles while maintaining good performance.
No memory effect: Unlike some other rechargeable batteries, liquid rechargeable Lithium-ion batteries do not exhibit a memory effect, meaning they do not require full discharge before recharging.
Portable electronics: Smartphones, laptops, tablets, and other portable electronic devices commonly utilize liquid rechargeable Lithium-ion batteries due to their high energy density and long life cycle.
Electric vehicles: Electric cars, motorcycles, and bicycles often employ liquid rechargeable Lithium-ion batteries as their primary power source due to their ability to provide sustained power output.
Aerospace applications: In military and aerospace applications, liquid rechargeable Lithium-ion batteries are used for their high energy density and reliability in extreme conditions.
In summary, liquid rechargeable Lithium-ion batteries are versatile and reliable power sources that find applications in various fields due to their unique composition, working principle, and characteristics.
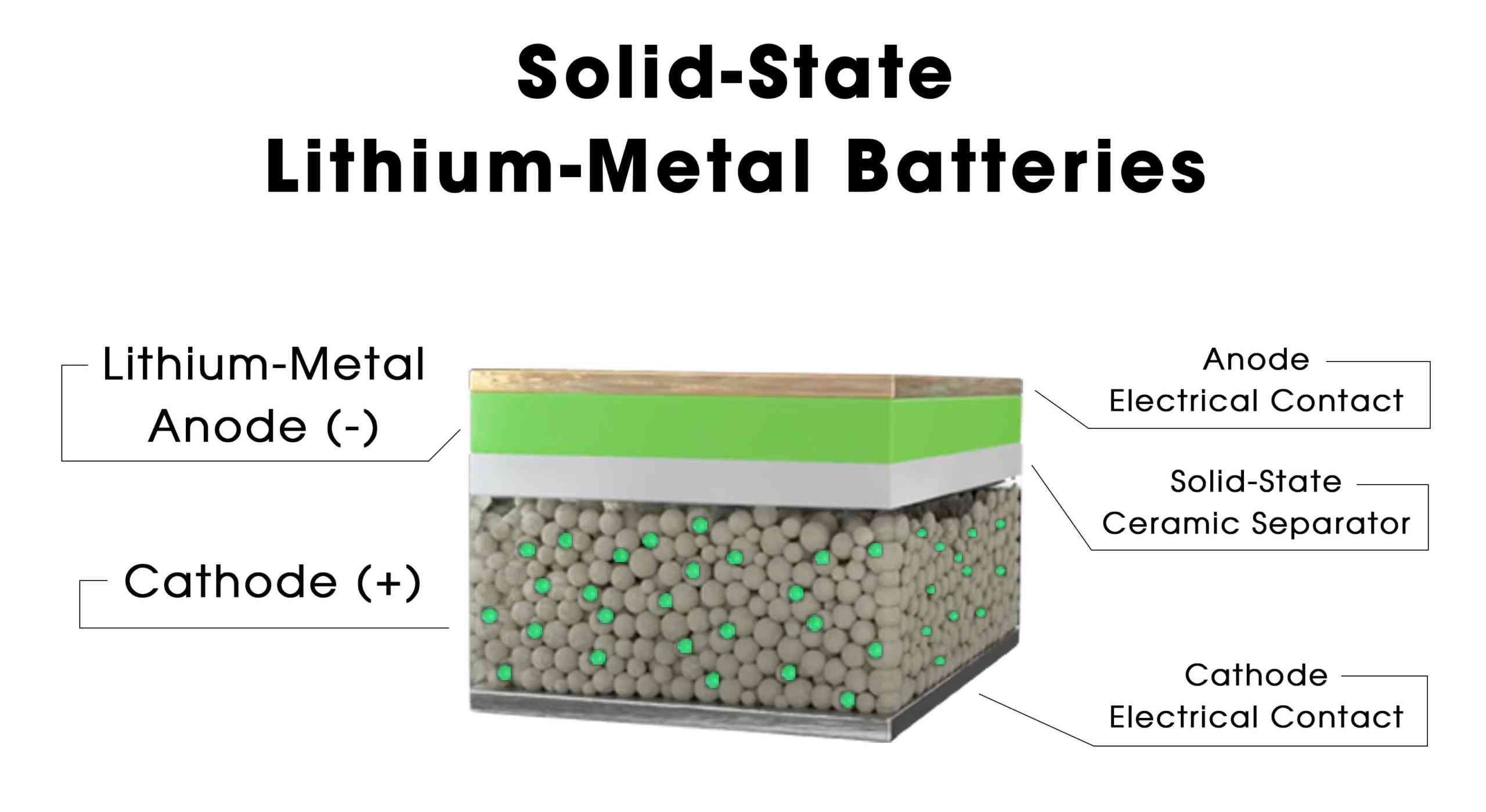
The all solid state rechargeable Lithium-ion battery, as its name suggests, utilizes solid electrolytes instead of liquid or gel electrolytes. This technology promises significant advantages over traditional Lithium-ion batteries, particularly in terms of safety, energy density, and longevity. The defining characteristic of the all solid state rechargeable Lithium-ion battery is its solid electrolyte, which eliminates the risk of electrolyte leakage or combustion, thus greatly enhancing the battery's safety. Furthermore, solid electrolytes tend to have higher lithium-ion conductivity compared to liquid electrolytes, potentially leading to improved performance and faster charging speeds. Additionally, solid electrolytes are not susceptible to evaporation or degradation over time, resulting in longer battery lifespans and better durability.
The half-solid state Li-ion battery is a type of Lithium-ion battery that combines features of both solid-state and liquid-state electrolytes. Specifically, it refers to a battery in which either one of the electrodes does not contain liquid electrolyte, while the other electrode does, or a battery where the solid electrolyte accounts for approximately half of the total electrolyte mass or volume within the cell. The defining characteristic of the half-solid state Li-ion battery lies in its hybrid electrolyte configuration. This design aims to balance the advantages and disadvantages of solid and liquid electrolytes. On one hand, the solid electrolyte component provides improved safety, stability, and potentially higher energy density compared to traditional liquid electrolytes. On the other hand, the liquid electrolyte component retains some of the advantages of liquid-state batteries, such as good ionic conductivity and compatibility with existing battery manufacturing processes. The half-solid state Li-ion battery offers several potential benefits. First, by incorporating a solid electrolyte, it significantly reduces the risk of electrolyte leakage and combustion, enhancing the battery's safety. Second, the solid electrolyte may enable the use of novel electrode materials, such as lithium metal, that are incompatible with liquid electrolytes but offer higher energy densities. Third, the hybrid electrolyte design allows for efficient ion transport while maintaining stability and safety. In summary, the half-solid state Li-ion battery represents a promising technology that aims to combine the benefits of solid-state and liquid-state electrolytes. Its hybrid electrolyte configuration offers improved safety, stability, and potential for higher energy densities compared to traditional Lithium-ion batteries, making it a compelling option for various applications requiring high-performance and reliable power sources.
A gel polymer rechargeable Li-ion battery is a type of Lithium-ion battery in which the liquid electrolyte is combined with polymer macromolecules to form a gel-state electrolyte. Currently, soft-packed Lithium-ion batteries encapsulated with plastic films are also referred to as polymer Lithium-ion batteries or simply polymer Li-ion batteries. In a gel polymer electrolyte Lithium-ion battery, the gel polymer electrolyte exists in the form of a gel within the separator and the electrodes. The defining characteristic of gel polymer electrolytes lies in their unique combination of liquid and solid properties. They possess some characteristics of a solid, such as mechanical strength and shape retention, while also exhibiting fluid-like properties that enable ion transport. This gel state electrolyte offers several advantages over traditional liquid electrolytes. It is more stable, reducing the risk of electrolyte leakage, and provides better safety. Additionally, gel polymer electrolytes tend to have higher ionic conductivity, leading to improved battery performance. The composition of gel polymer electrolytes typically includes a liquid electrolyte, such as an organic solvent containing lithium salts, and a polymer matrix. The polymer matrix acts as a scaffold, immobilizing the liquid electrolyte and forming the gel state. This gel structure allows for efficient ion transport while maintaining the stability and safety benefits of a solid electrolyte.
A pouch Li-ion cell is a Lithium-ion battery that utilizes a flexible plastic laminate film as its outer packaging, rather than a rigid metal casing. This design choice offers several advantages over traditional metal-cased batteries. First and foremost, the use of a plastic laminate film enables the pouch cell to have a significantly lighter weight compared to metal-cased batteries. This reduced weight is especially beneficial in applications where weight savings are critical, such as in electric vehicles and portable electronics. Additionally, the flexible nature of the plastic laminate film allows the pouch cell to conform to irregular shapes and spaces, providing greater design flexibility. This adaptability is useful in applications where space is limited or where the battery needs to fit into a specific form factor. Furthermore, the plastic laminate film used in pouch cells often has excellent sealing properties, which helps to prevent electrolyte leakage and improves the battery's safety. This is achieved through the use of heat-sealing techniques that create strong and reliable seals around the edges of the cell. In terms of performance, pouch Li-ion cells are comparable to their metal-cased counterparts. They utilize the same lithium-ion chemistry and electrode materials, allowing them to achieve similar energy densities, discharge rates, and cycle life.
The pouch Li-ion cell is commonly found in numerous consumer electronics and applications in our daily lives. For instance, it is a popular choice for smartphones, tablets, and other portable devices due to its lightweight and compact design. The flexible nature of the plastic laminate film allows the battery to conform to the internal contours of these devices, maximizing space utilization and minimizing overall weight. Additionally, the pouch Li-ion cell is also utilized in wearable technology such as smartwatches and fitness trackers, where its thin and flexible form factor is essential for comfortable wearability.




The lab focuses on solid-state battery research to overcome traditional lithium batteries' safety and energy density issues, supporting environmental sustainability. It develops innovative solid-state electrolytes, refines electrode materials, and investigates ion transfer and interface stability to revolutionize battery technology.

The electric vehicle battery industry is rapidly developing, focusing on technological innovation, market competition, and sustainability. Research hotspots include solid-state batteries, new types of electrolytes, BMS optimization, and recycling technologies. The environmental adaptability, safety, and economic viability of batteries are key research areas, and the industry is expected to undergo more innovation and transformation.

Specializing in battery preparation technology research, the focus is on overcoming existing energy storage challenges by innovating in electrode materials, battery chemistry, and manufacturing processes to improve performance, safety, and reduce costs. Sustainability and recycling technologies for batteries are also emphasized to mitigate environmental impacts and foster the growth of green energy.