Electrochemical impedance spectroscopy (EIS) is a powerful technique used to analyze the variation of AC impedance with frequency in an electrochemical system under a polarization steady-state condition, typically at the equilibrium potential. This method provides valuable information about the electrochemical processes, such as charge transfer resistance, electrolyte resistance, and double-layer capacitance, occurring within the system.
During the EIS testing process, several issues may arise, and appropriate measures should be taken to address them:
1.Electrode Polarization: Electrode polarization can significantly affect EIS measurements. Polarization can arise due to various factors, including impedance from electrolyte resistance, charge transfer resistance, and double-layer capacitance. To mitigate this issue, it is crucial to apply proper calibration methods, such as subtracting the contribution of electrode polarization from the impedance data, to obtain accurate results.
2.Experimental Setup: The experimental setup for EIS testing should be carefully designed to minimize unwanted parasitic elements that can introduce errors in measurements. Factors such as cable capacitance, contact resistance, and stray capacitance can affect the accuracy of impedance measurements. Appropriate shielding, careful electrode placement, and the use of high-quality cables and connectors can help minimize these effects.
3.Frequency Range and Resolution: Selecting an appropriate frequency range and resolution is essential for obtaining meaningful and accurate impedance spectra. The frequency range should cover the relevant electrochemical processes of interest, and the resolution should be sufficient to capture the details of impedance changes. Choosing an excessively wide or narrow frequency range may result in insufficient sensitivity or loss of relevant information. Therefore, careful consideration of the characteristics of the system and expected processes is necessary to determine the optimal frequency range and resolution.
4.Data Interpretation: Interpreting the impedance spectra obtained from EIS measurements requires expertise and knowledge of electrochemical principles. The impedance spectra are typically fitted to equivalent circuit models to extract meaningful information about the properties and processes of the system. Proper model selection and fitting techniques are essential to accurately interpret the impedance spectra and extract relevant parameters.
The significance of electrochemical impedance spectroscopy lies in its ability to provide a detailed understanding of the electrochemical processes occurring within a system. EIS enables the determination of various electrochemical parameters, such as charge transfer resistance, diffusion coefficients, and reaction kinetics. It aids in the characterization of electrochemical interfaces, the evaluation of electrochemical system performance, and the identification of degradation mechanisms.
EIS finds applications in various fields, including battery research, fuel cell analysis, corrosion studies, and sensor development. It helps in the optimization of electrode materials, the investigation of electrochemical reaction mechanisms, and the development of efficient energy storage and conversion devices. EIS also enables the evaluation of the effects of environmental factors, such as temperature, humidity, and gas composition, on electrochemical systems.
In summary, electrochemical impedance spectroscopy is a valuable technique for studying the variation of AC impedance with frequency in an electrochemical system under a polarization steady-state condition. By addressing potential issues and applying appropriate data analysis techniques, this method contributes to a deeper understanding of electrochemical processes, system optimization, and the development of advanced electrochemical technologies.
AC Voltammetry is a technique used to study the relationship between the amplitude and phase of AC current and the DC polarization potential at a specific frequency. This method provides valuable information about the electrochemical processes occurring at the electrode-electrolyte interface.
The significance of AC Voltammetry lies in its ability to provide insights into the electrochemical processes occurring at the electrode-electrolyte interface. By studying the relationship between AC current amplitude, phase, and DC polarization potential, this technique enables the determination of various electrochemical parameters, such as charge transfer kinetics, double-layer capacitance, and faradaic processes.
AC Voltammetry finds applications in diverse fields, including corrosion studies, electroplating, battery characterization, and sensor development. It helps in the evaluation of electrode performance, optimization of electrochemical processes, and understanding the mechanisms of electrochemical reactions. AC Voltammetry also aids in the development of new materials and technologies for energy storage, electrocatalysis, and electrochemical sensing.
In summary, AC Voltammetry is a valuable technique for investigating the relationship between AC current characteristics and DC polarization potential at a specific frequency. By addressing potential issues and applying appropriate data analysis techniques, this method contributes to a deeper understanding of electrochemical processes, system optimization, and the development of advanced electrochemical applications.




Technology
December 04, 2025

The lab focuses on solid-state battery research to overcome traditional lithium batteries' safety and energy density issues, supporting environmental sustainability. It develops innovative solid-state electrolytes, refines electrode materials, and investigates ion transfer and interface stability to revolutionize battery technology.

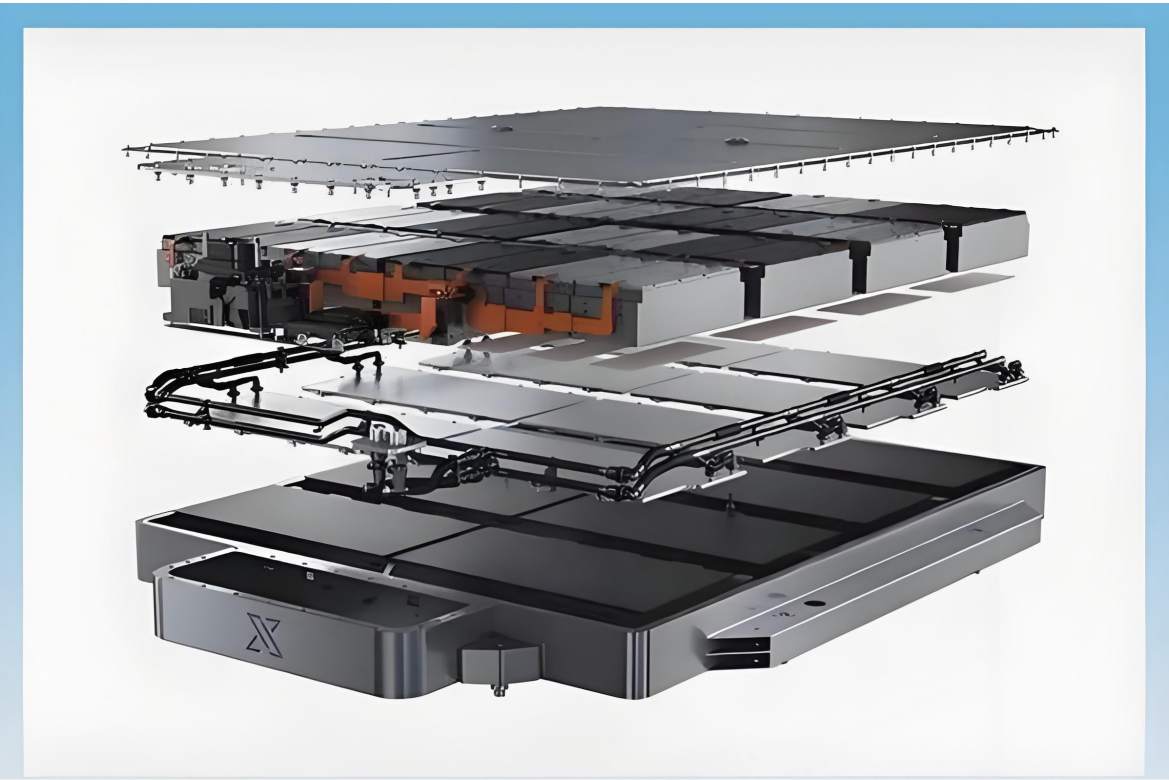
The electric vehicle battery industry is rapidly developing, focusing on technological innovation, market competition, and sustainability. Research hotspots include solid-state batteries, new types of electrolytes, BMS optimization, and recycling technologies. The environmental adaptability, safety, and economic viability of batteries are key research areas, and the industry is expected to undergo more innovation and transformation.

We specialize in battery preparation technology research, focusing on overcoming existing energy storage challenges by innovating in electrode materials, battery chemistry, and manufacturing processes to improve performance, enhance safety, and reduce costs. Sustainability and recycling technologies for batteries are also emphasized to mitigate environmental impacts and foster the growth of green energy.