Since the "double carbon" goal was put forward, the low-carbon trend of global energy and industrial development has been formed. Therefore, in order to reduce CO2 emissions and achieve the goal of carbon neutrality, it is of great significance to use clean energy to drive vehicles. Due to its high energy density, long cycle life, low cost and low environmental pollution, electric vehicles powered by lithium-ion batteries have gradually become the focus of attention.
However, so far, although the number of global electric vehicles is expected to reach 230 million by 2030, its market penetration and consumer acceptance are still low. One of the important reasons is mileage anxiety. Therefore, fast charge lithium-ion batteries have become a research hotspot in recent years.
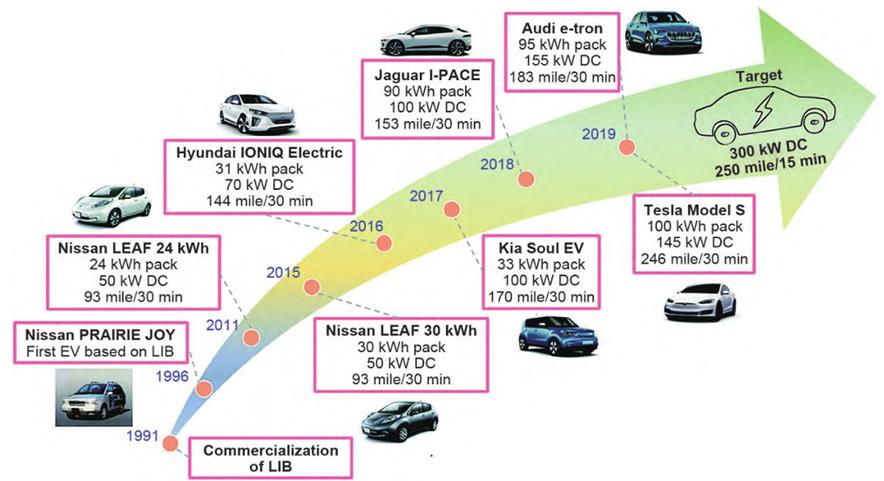
Figure-1-The-development-history-of-lithium-ion-battery-electric-vehicles-and-the-corresponding-fast-charging-capacity
According to the definition of the United States Advanced Battery Consortium (USABC), fast charging is to obtain 80 % of the state of charge (SOC) of the battery in 15 minutes, that is, to charge the battery pack to 80 % at a rate of 4 C. At the same time, fast charge lithium-ion batteries must be evaluated simultaneously on three indicators: 1.Charging time; 2.The obtained specific energy; 3.The number of cycles at high rate. Although lithium-ion battery electric vehicles have made significant progress in fast charging (Figure 1), there is still a gap from the target.
Lithium-ion batteries are also known as 'rocking chair' batteries, in which Li+ moves between the positive electrode and the negative electrode. The key factor affecting the performance of the battery is the transport of lithium ions, including the diffusion of Li+ in the electrode material, the transport of Li+ in the solid electrolyte intermediate phase and the transport of Li+ in the electrolyte intermediate. In the process of rapid charge and discharge, the electrochemical process and the structure of the battery itself will affect the charge transfer of ions and electrons in the whole process, thus affecting its performance. The influence of fast charging technology on lithium batteries can be mainly reflected in the following aspects:
1.Lithium plating: Among all the factors that lead to the performance degradation of lithium-ion batteries under fast charging, the most unfavorable factor is the lithium plating on the surface of the graphite negative electrode (Figure 2). During the charging process, Li+ migrates from the positive electrode to the negative electrode and inserts into the graphite layer; however, under fast charging conditions, the transmission rate of Li+ in the electrolyte is much faster than that of Li+ embedded in the graphite layer, and more Li+ accumulates on the surface of the negative electrode rather than embedded in the gap of the graphite atomic layer, resulting in severe voltage polarization and the potential of the graphite negative electrode is reduced to 0 V (vs. Li/Li+). The deposited lithium will further react with the electrolyte to form an ineffective SEI layer or a 'dead' lithium film isolated from the anode. The high impedance will not only increase the internal resistance of the battery, but also reduce the energy density of the battery, resulting in accelerated attenuation of battery capacity.
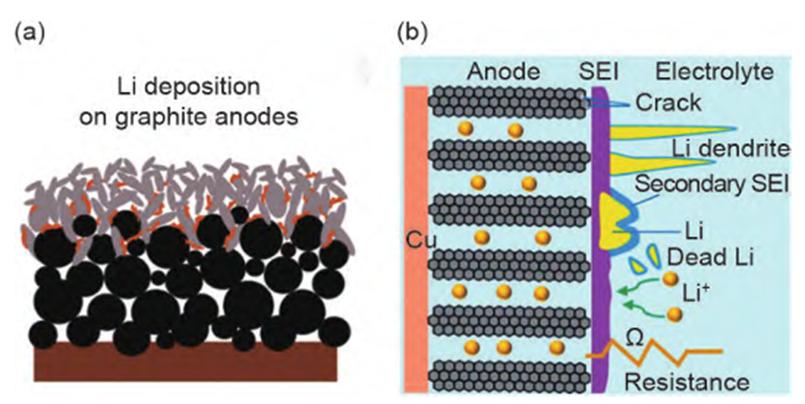
Figure-2-Graph-of-lithium-plating-on-graphite-anode-under-fast-charge-condition-and-its-degradation-mechanism
2.Mechanical effect: According to different scales, mechanical degradation can be divided into the separation of electrode particles, conductive materials and adhesives, the fracture inside the electrode particles, the separation between the active material and the current collector, and the delamination between the electrode sheets. The main reason for these phenomena is that the gradient distribution of lithium concentration during fast charging causes uneven stress between components. During the fast charging process, Li+ is quickly removed from the positive electrode and embedded in the negative electrode, resulting in a serious strain mismatch between Li+ and different parts of the electrode particles. When the energy release rate or stress intensity coefficient exceeds a certain value, the crack will propagate in the particles, resulting in the cracking of SEI/CEI. The effects of mechanical degradation on battery performance can be divided into active material loss, lithium storage loss and impedance growth.
3.Thermal effect: The heating problem caused by high charging rate will lead to the performance degradation of the battery. The high temperature in the battery will accelerate many side reactions, including material phase change, gas release, binder decomposition and metal dissolution. In particular, the lattice expansion caused by high temperature aggravates the volume expansion and leads to mechanical stress and even particle cracking. The electrolyte may also be thermally decomposed as the temperature increases, and the resulting gaseous by-products will also aggravate the mechanical stress. In addition, the researchers found that as the charging rate increases, the thermal stability of the battery decreases. Thermal runaway usually causes smoke, fire and even explosion, which poses a great threat to the safety of the battery.
4.Others: In the fast charging process of the battery, the polarization will increase significantly, which will affect the specific capacity, energy density and cycle life of the battery.
In order to further optimize the fast charging performance of the battery, researchers have made progress in many aspects. The optimization strategy is mainly carried out from the following aspects:
1.The optimization of the negative electrode: For the negative electrode, ensuring the rapid diffusion of Li+ in the bulk phase of the negative electrode and reducing the interface kinetic barrier between the negative electrode and the electrolyte are the key to achieve rapid charging. If the Li+ transport kinetics cannot meet the requirements of fast charging, the polarization on the negative electrode will lead to lithium plating, which will reduce the cycle life and even cause safety problems. Therefore, improving the Li+ transport rate and electron transport kinetics is an effective way to achieve fast charging.
2.Optimization of cathode materials: The commonly used cathode materials for lithium ion batteries include LiCoO2, LiFePO4 and ternary materials (LiNixCoyAlzO2 and LiNixCoyMnzO2). Among them, the ternary material has high specific capacity, high voltage platform and good cycle performance. It is a promising cathode material for fast charging lithium-ion batteries, but its safety needs to be improved. The ideal cathode material should have fast Li+ deintercalation ability and stable cycle performance. Therefore, the current research focus is on improving the performance of commonly used cathode materials by modification, and developing new fast-charging cathode materials.
3.Optimization of electrolytes: The traditional lithium-ion battery electrolyte is mainly composed of lithium salts and organic solvents, and its composition and solvation structure affect the transport kinetics of Li+ in the electrolyte and SEI/CEI. At the same time, during the charge and discharge process, in addition to the delithiation and lithium insertion reactions in the electrode material, Li+ completes the solvation and desolvation process on the electrode surface and participates in the formation of the SEI/CEI structure. The properties of the SEI layer will significantly affect the rate performance of the battery. In addition, the side reaction caused by fast charging will reduce the stability of the electrolyte, and the generation of internal heat or the growth of lithium dendrites will reduce the conductivity of the electrolyte and trigger an exothermic reaction. Therefore, the development of electrolytes that meet fast charging performance and high safety has become a research hotspot.
1.Optimization of anode: Taking graphite anode material as an example, it is the most commonly used carbon-based anode material for lithium ion batteries. It has a layer spacing of 0.34 nm, allowing Li+ to be reversibly intercalated and exfoliated. At the same time, the graphite potential is very close to the redox potential of lithium, which can make the battery exhibit higher energy density. However, graphite has slow lithium insertion kinetics and low lithiation voltage (0.08 V vs. Li/Li+). The large polarization under high current conditions pushes the graphite potential to the threshold of lithium metal deposition (0 V vs. Li/Li+), resulting in lithium plating on the graphite surface.
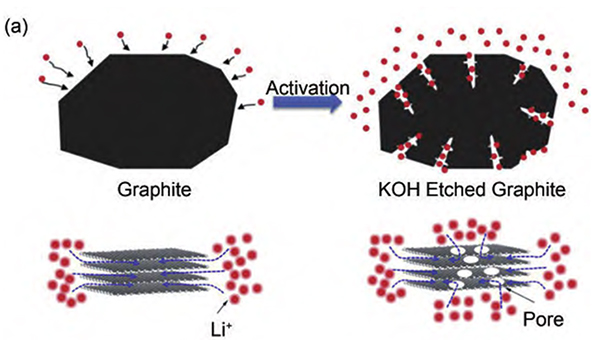
Figure-3-Schematic-diagram-of-original-graphite-and-KOH-etched-graphite
In order to solve this problem, a research team proposed to etch graphite with KOH to form holes on its surface that can reduce the diffusion distance of Li+ to improve the charge and discharge rate capability (Figure 3). The capacity retention rate of KOH-etched graphite at 3 C charge and discharge is 93%. Even at a higher cycle rate of 6 C, it can still exhibit a capacity retention rate of 74% (Figure 4).
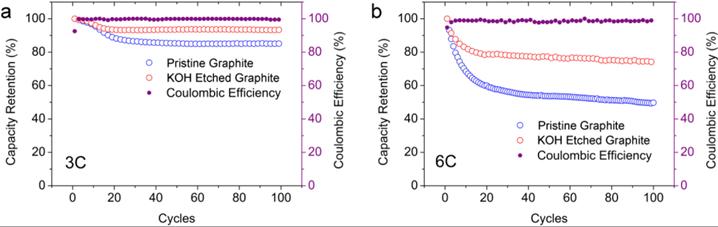
Figure-4-Comparison-of-electrochemical-performance
2.Optimization of the cathode: The use of element doping to improve the performance of the cathode has received extensive attention. It has been reported that element doping can change the lattice size, expand the Li+ diffusion channel, inhibit the strain caused by the change of lattice volume during the cycle, and improve the mechanical stability.
To this end, a research team has doped Mg and Ti into LCO materials. At the same time, Mg2+ and Ti4+ co-doping can also improve the rate performance and high-pressure cycle stability of LCO cathodes. Specifically, Mg2+ and Ti4+ optimize the particle size distribution and reduce the charge transfer resistance, thereby increasing the diffusion coefficient of Li+ in the cathode. As shown in Figure 5, the co-doped sample exhibits an initial discharge capacity of 179.6 mAh/g in the voltage range of 2.75~4.5 V at 1 C. After 100 cycles, the capacity retention rate of the sample can reach 82.6%. In addition, the co-doped sample shows better rate performance, with a high discharge capacity of 151.4 mAh/g at 5 C.
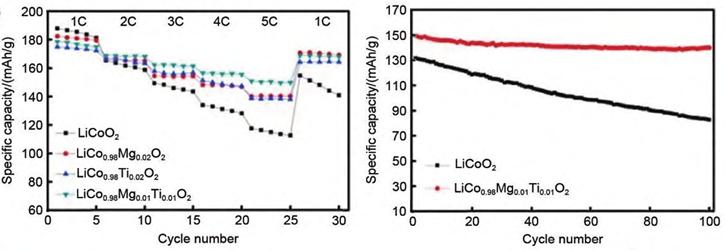
Figure-5-Electrochemical-cycling-performance-of-different-LCO-materials
3.Optimization of electrolytes: High concentration electrolytes (usually>3 mol/L) can achieve better lithium-ion batteries by adjusting the solvation structure of Li+. The evolution of the solvent structure of typical high concentration electrolytes at different lithium salt concentrations is shown in Figure 6.
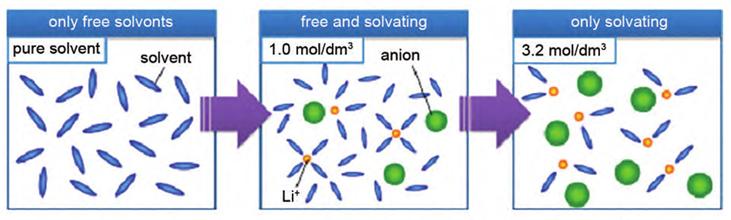
Figure-6-The-schematic-diagram-of-coordination-structure-in-low-concentration-electrolyte-and-high-concentration-electrolyte
In order to achieve the purpose of fast charging, a local high-concentration electrolyte composed of LiFSI, bis(2,2,2-trifluoroethyl) ether (BTFE) and dimethoxyethane (DME) was proposed by the scientific research team. In this system, the free solvent molecules disappear and contact ion pairs (CIPs) and aggregates (AGGs) are formed. The anions driven by CIPs and AGGs achieve their decomposition voltage priority decomposition earlier than the solvent molecules, and a uniform and strong LiF-rich SEI can be formed on the surface of graphite, which can significantly inhibit the co-embedding of solvents in graphite. Highly reversible Li+ insertion/extraction is achieved. As shown in Figure 7, the C/Li battery exhibits the potential for fast charging (a high capacity of 220 mAh/g at 4 C) and excellent cycle stability (85.5 % capacity retention after 200 cycles at 4 C).
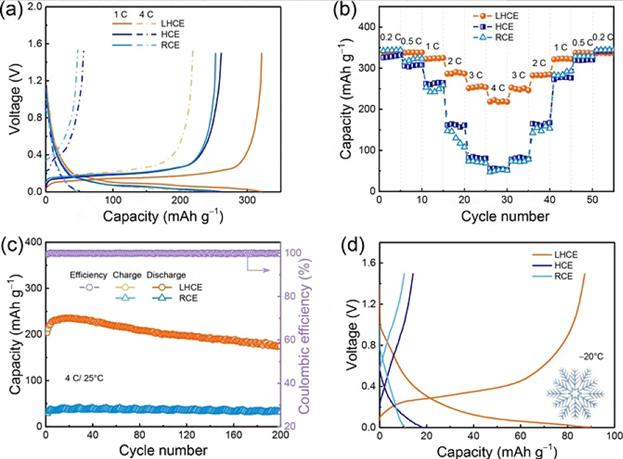
Figure-7-Comparison-of-electrochemical-properties-of-different-electrolytes
NEWARE
4130 164TH CT SE, Bellevue, WA, USA, 98006




● Voltage&Current Accuracy:±0.01% F.S.
● Recording Frequency:100Hz
● Current Response Time:≤1ms
● Minimum Pulse Width:500ms
● Off-Line Test:1GB/CH
● Cycle Life, GITT Test, DCIR Test, dQ/dV Curve

● Voltage & Current Accuracy:±0.01% F.S.
● Recording Frequency:10Hz
● Sampling Time:100ms
● Current Response Time:≤1ms
● Minimum Pulse Width:500ms
● Off-Line Test: 1GB

● Voltage & Current Accuracy:±0.05% F.S.
● Recording Frequency:10Hz
● Sampling Time:100ms
● Current Response Time:≤1ms
● Energy Efficiency:>65%
● Off-Line Test: 1GB

● Voltage & Current Accuracy:±0.05% F.S.
● Recording Frequency:10Hz
● Sampling Time:100ms
● Current Response Time:≤1ms
● Energy Efficiency:>65%
● Off-Line Test: 1GB

● Voltage & Current Accuracy:±0.05% F.S.
● Recording Frequency:10Hz
● Sampling Time:100ms
● Current Response Time:≤1ms
● Energy Efficiency:>65%
● Off-Line Test: 1GB

● Voltage Accuracy:±0.02% F.S.
● Current Accuracy:±0.05% F.S.
● Resolution Ratio AD/DA:16bit
● Current Response Time:≤1ms
● Minimum Pulse Width:100ms
● Off-Line Test:1GB/CH

● Voltage & Current Accuracy:±0.05% F.S.
● Recording Frequency:100Hz
● Current Conversion Time:≤6ms
● Current Response Time:≤3ms
● Minimum Pulse Width:100ms
● Feedback Efficiency (Max) :75%

● Voltage & Current Accuracy:±0.02% F.S.
● Voltage & Current Stability:±0.01% F.S.
● Recording Frequency:1000Hz
● Resolution AD:16bit
● Current Response Time:≤100μs
● Off-Line Test: 1GB