With the advancement of technology and the development of society, energy consumption and environmental pollution problems are becomingmore and more serious, which has led to the pursuit of efficient and environmentally friendly energy storage solutions. Lithium-ion battery is one of the most mainstream energy storage devices, and its performance improvement has become a research hotspot.
However, the traditional methods of improving the performance of electrode materials and developing new electrolyte systems to improve battery performance have been limited. During the battery cycle, the irreversible loss of lithium ions will seriously affect the energy density and cycle life of the battery. The previous battery performance improvement methods have been unable to meet people's current demand for high energy density. Therefore, prelithiation technology came into being.
The prelithiation technology is the method of adding a small amount of lithium source to the battery system before the battery is charged/discharged, which can be used to make up for the lithium consumption during the battery charge/discharge reaction. Up to now, prelithiation technology is mainly divided into three categories: electrochemical prelithiation, chemical prelithiation and adding sacrificial lithium additives.
1.Electrochemical prelithiation: It is the prelithiation method for cathode/anode of lithium-ion batteries. The core is to complete the prelithiation of the electrodes by controlling the depth of electrochemical charge and discharge. Specifically, electrochemical prelithiation involves the formation of a system of lithium foil, electrolyte, and electrode, which allows lithium ions to actively diffuse through the electrolyte to the positive/negative electrode and complete the prelithiation process through an applied voltage. This method can be regarded as a precise control version of the lithium foil direct contact method, in which the parameters such as the degree and speed of prelithiation can be adjusted.
Electrochemical prelithiation can be divided into two methods: ex-situ and in-situ electrochemical prelithiation. In the process of ex-situ electrochemical prelithiation, it is necessary to assemble the electrode to be prelithiated with lithium metal into a half-cell. After a specific charge and discharge cycle, the electrode reaches the set prelithiation level, and then the prelithiated electrode is assembled with the new positive/negative electrode into a full battery. The in-situ electrochemical prelithiation requires the redesign of the lithium-ion battery. In the assembly process, lithium metal is added as the third electrode in advance, and then the prelithiation process is completed. Among them, Figure 1 is the schematic diagram of electrochemical prelithiation.
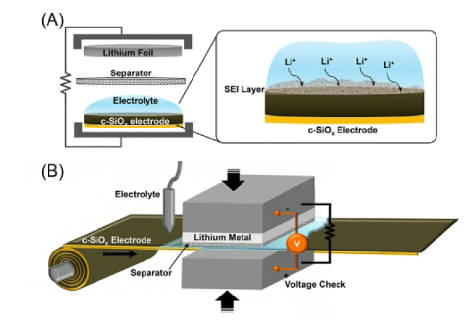
Figure-1-The-schematic-diagram-of-electrochemical-prelithiation
2.Chemical prelithiation: It mainly includes chemical synthesis prelithiation, direct contact prelithiation and chemical solution prelithiation. In contrast, the research and application of direct contact prelithiation and chemical solution prelithiation are more extensive.
Direct contact prelithiation is mainly the prelithiation method for the anode of lithium-ion battery, that is, the electrode to be prelithiated is directly pressed and contacted with the lithium foil to complete the prelithiation process. Although the process is simple to operate, there are still problems such as difficulty in controlling the amount of pre-embedded lithium and difficulty in effectively separating lithium foil. Among them, Figure 2 is the schematic diagram of direct contact prelithiation.
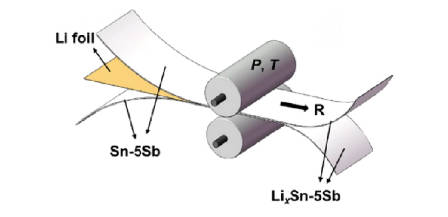
Figure-2-The-schematic-diagram-of-direct-contact-prelithiation
Chemical solution prelithiation is the use of highly reducible lithium-containing solution to treat the electrode, and the active lithium is transported to the electrode material during the redox reaction. In this method, most of the chemical prelithiation reactants are lithium-containing organic compounds, such as biphenyl lithium, naphthalene lithium, butyl lithium, etc. Chemical prelithiation can achieve relatively high uniformity, and the degree of prelithiation can be controlled by controlling the treatment time. The treatment time is positively correlated with the degree of prelithiation. Therefore, its application scenarios are more extensive. Figure 3 is the schematic diagram of prelithiation of chemical solutions.
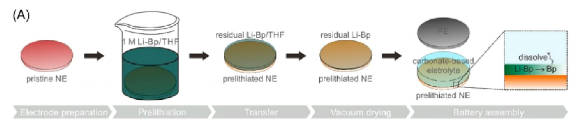
Figure-3-The-schematic-diagram-of-prelithiation-of-chemical-solutions
3.Sacrificial lithium-compensation additives: This method is to add lithium-rich lithium-containing additives on the positive/negative side. The additive will complete irreversible decomposition at the beginning of the cycle, thereby releasing lithium ions to compensate for lithium loss in the battery system.
According to the characteristics of the additive itself, it will be divided into positive side lithium compensation additive and negative side lithium compensation additive, and this kind of additive has some common characteristics and requirements, such as:
(1) It has high irreversible specific capacity and low decomposition voltage;
(2) It has good air stability and safety;
(3) less decomposition residues, less gas output;
(4) It has good process compatibility and cost-effectiveness.
Based on the above characteristics, dozens of additives have been developed to meet different production needs. Figure 4 is the typical lithium compensation additive.
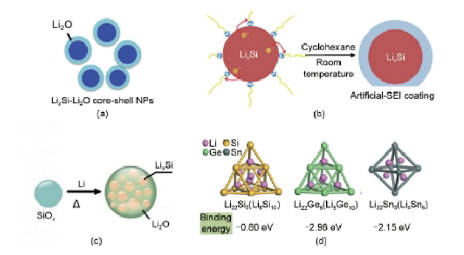
Figure-4-The-typical-lithium-compensation-additive
In view of the existing classification of prelithiation technology, a case will be selected for introduction and analysis:
1.Electrochemical prelithiation: The prelithiation process is usually to assemble the prelithiated electrode with lithium metal into a half-cell, and then perform electrochemical discharge (and subsequent cycle) treatment to complete the prelithiation process. After prelithiation, the SEI film is usually formed with a certain amount of lithium embedded in the anode, which will effectively improve the reversible capacity and energy density of the subsequent battery. As shown in Figure 5, the constant current charge-discharge curve of the original Gr||original LNMO full battery and the prelithiated Gr||original LNMO full battery shows that the initial discharge specific capacity of the full battery has been significantly improved by prelithiation of the negative electrode.
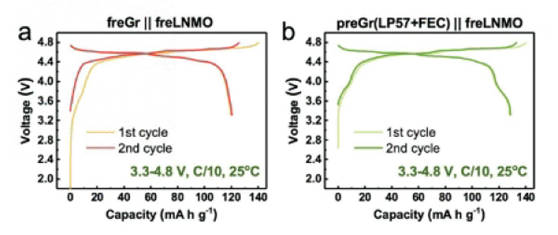
Figure-5-Comparison-of-charge-discharge-curve-of-full-cell-before-and-after-electrochemical-prelithiation
2.Chemical prelithiation: Taking the prelithiation of chemical solution as an example, this method usually involves the preparation of the solution, the immersion of the electrode, the cleaning of the electrode and the subsequent battery assembly. As shown in Figure 6, taking Bp-Li-2MT solution as an example, by adjusting the prelithiation time (i.e., the time of solution immersion), the amount of lithium embedded in graphite and the coulombic efficiency in the first cycle can be effectively controlled. Using prelithiated graphite to assemble the full battery, the initial reversible capacity of the full battery has been significantly improved.
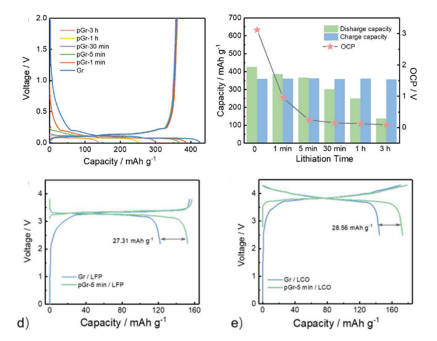
Figure-6-Comparison-of-the-effects-of-chemical-prelithiated-graphite
3.Sacrificial lithium-compensation additives: These additives are usually lithium-rich substances. So far, dozens of substances have been developed and successfully applied to the prelithiation system, such as lithium oxide, lithium nitride, lithium carbonate, lithium oxalate, lithium-silicon alloy, etc.
As shown in Figure 7, it is a Li2O-type lithium compensation additive. By adding a transition metal M to the additive, the decomposition voltage can be effectively reduced to complete the decomposition at a lower potential. The irreversible lithium ions released by the additive will compensate for the lithium loss during the battery cycle.
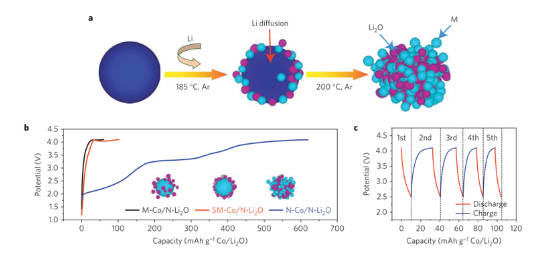
Figure-7-The-synthesis-diagram-and-test-result-of-M+Li2O-additive
As an important means to improve the performance of lithium-ion batteries, prelithiation technology has broad prospects for future development. First of all, with the increase of new energy demand, the application of prelithiation technology in the field of electric vehicles and renewable energy storage will be more popular, which will promote the further improvement of battery energy density and cycle life. Secondly, advances in materials science will promote the development of new electrode materials, such as lithium-containing compounds with high capacity and high stability, which will enhance the overall performance of the battery. At the same time, the introduction of process optimization and intelligent manufacturing technology can improve production efficiency, reduce production costs, and make prelithiation technology more competitive in commercial applications. In addition, research on environmentally friendly materials and processes will also become the focus of future development to meet the requirements of sustainable development. With the development of artificial intelligence and big data technology, the intelligent monitoring system will provide real-time data support for the prelithiation process to ensure the safety and reliability of the battery.
Based on the existing research content, the future development direction of prelithiation technology may focus on the following aspects:
1.Material innovation: Further research and development of new high-performance lithium-containing materials to improve battery energy density and cycle life;
2.Process optimization: Improve the current production process of prelithiation, reduce costs, improve safety and production efficiency, and ensure the feasibility of technology in large-scale applications;
3.Intelligent monitoring: develop a real-time monitoring system to track the state of the battery during the pre-lithiation process to ensure safety and stability;
4.Environmental friendliness: Explore green chemical methods to reduce the impact of prelithiation on the environment and promote sustainable development;
5.Application expansion: The prelithiation technology is applied to more fields, such as electric vehicles, energy storage systems and portable electronic devices, to meet the growing market demand.




The lab focuses on solid-state battery research to overcome traditional lithium batteries' safety and energy density issues, supporting environmental sustainability. It develops innovative solid-state electrolytes, refines electrode materials, and investigates ion transfer and interface stability to revolutionize battery technology.

The electric vehicle battery industry is rapidly developing, focusing on technological innovation, market competition, and sustainability. Research hotspots include solid-state batteries, new types of electrolytes, BMS optimization, and recycling technologies. The environmental adaptability, safety, and economic viability of batteries are key research areas, and the industry is expected to undergo more innovation and transformation.

We specialize in battery preparation technology research, focusing on overcoming existing energy storage challenges by innovating in electrode materials, battery chemistry, and manufacturing processes to improve performance, enhance safety, and reduce costs. Sustainability and recycling technologies for batteries are also emphasized to mitigate environmental impacts and foster the growth of green energy.