In the past few decades, lithium-ion batteries (LIBs) have emerged as one of the most prominent energy storage technologies, supporting advancements in mobile devices, electric vehicles, and large-scale energy storage systems. Adhesives play a critical role in the performance, stability, and lifespan of LIBs by securely bonding active materials, conductive additives, and current collectors to form stable electrode structures, ensuring excellent electrochemical performance and cycling stability. Below are the adhesive bonding mechanisms and commonly used types.
Polymer adhesives create bridges between current collectors, conductive carbon,and active materials to maintain electrode integrity. During adhesion, polymers initially adhere and wrap around different components' surfaces, then penetrate electrode particle pores under solvent action, solidifying through drying or polymerization. Adsorption on particle surfaces forms boundary layers, curing layers, and free layers, where the curing and free layers' performance primarily depends on the adhesive's intrinsic properties. Currently, six different theories about the adhesive mechanism of polymer adhesives have been established: mechanical interlocking theory, electrostatic theory, wetting theory, diffusion theory, chemical bonding theory, and weak boundary layer theory. In general, different theories complement each other, with mechanical interlocking theory and chemical bonding theory widely used to explain the bonding mechanism between polymers and matrices.
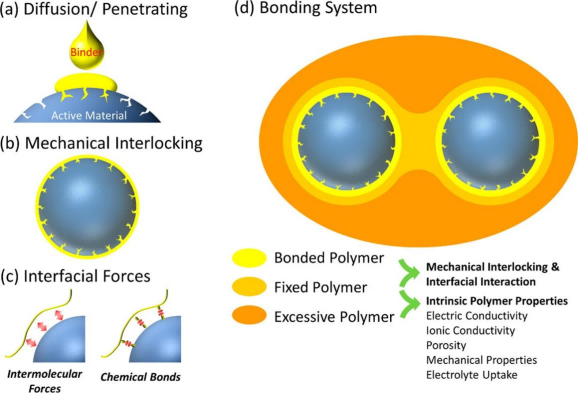
Figure 1 Schematic diagram of bonding mechanism of different binders
Polyvinylidene fluoride (PVDF): Due to its excellent chemical stability and mechanical properties, PVDF is one of the most commonly used positive electrode adhesives in lithium-ion batteries. It maintains stability over a wide voltage range during battery operation and does not react with electrolytes, ensuring long-term battery operation. However, PVDF is sensitive to environmental conditions (such as humidity), affecting its performance and bonding effectiveness. Moreover, PVDF is relatively costly compared to other adhesive types.
Adhesive mechanism: For conventional PVDF, the primary mechanism is van der Waals forces, which are intermolecular forces responsible for adhesion. Some modified PVDF variants have a dual mechanism: one part involves van der Waals forces due to the high molecular weight, and the other part involves chemical bonding with the foil material as a result of the modification.
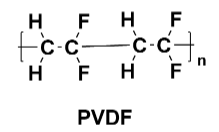
Figure 2 PVDF Structure
To overcome these drawbacks, researchers may explore the use of other types of binders or the modification of PVDF to improve its performance and reduce costs. In addition, the development of new types of binders is an important direction in the research of battery technology.
Polytetrafluoroethylene (PTFE), commonly known as Teflon, is a polymer material with very unique properties. PTFE exhibits extremely high resistance to almost all chemicals, including strong acids, strong bases, and organic solvents, making it very popular in the chemical industry and laboratory equipment. PTFE has excellent mechanical properties and can remain stable at extreme temperatures, with a continuous use temperature range typically between -200°C and 260°C, and can withstand even higher temperatures for short periods.
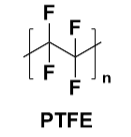
Figure 3 PTFE Structure
However, PTFE has very low surface energy, making it non-stick on most surfaces. While this property is advantageous in some applications, the low surface energy can lead to insufficient adhesion when PTFE is used as an adhesive.
Polyacrylic acid (PAA) and lithium polyacrylate (PAA-Li): These water-based adhesives are valued for their environmental friendliness and low cost. They provide good bonding strength through the formation of hydrogen bonds and physical entanglement, suitable for applications requiring increased production efficiency and reduced costs. However, their stability in high humidity environments is poor.
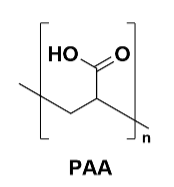
Figure 4 PAA Structure
Polyvinyl alcohol (PVA): As a water-based adhesive, PVA is used in the preparation of lithium-ion battery electrodes due to its good adhesive ability and environmental friendliness. PVA also enhances adhesion through hydrogen bonding, but its poor water resistance limits its use in some applications.
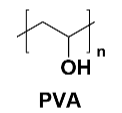
Figure 5 PVA Structure
Polyethylene oxide (PEO): PEO is widely used in the medical and biotechnology fields due to its good solubility and biocompatibility. It can form stable gels in aqueous solutions, suitable for drug delivery systems and tissue engineering. PEO’s non-toxicity and biodegradability make it ideal for biomedical materials. However, PEO's main drawback is its relatively low mechanical strength, which may limit its use in applications requiring high mechanical performance.
Binding mechanism: Conventional PEO primarily works through hydrogen bonds, where intermolecular hydrogen bond forces provide adhesion. Modified PEO can enhance interaction with substrates by introducing functional groups, thereby improving adhesion strength. For instance, chemically modified PEO with carboxyl or hydroxyl groups can form stronger chemical bonds with metals or inorganic materials.
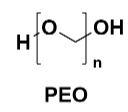
Figure 6 PEO Structure
However, the main drawbacks of PEO include its relatively low mechanical strength, which may limit its use in applications requiring high mechanical performance. Additionally, PEO gels may be unstable at high temperatures, limiting its use in applications requiring thermal stability.
Sodium carboxymethyl cellulose (CMC-Na): The most widely used and largest volume cellulose type in the world today, it is a cellulose derivative with a degree of polymerization of glucose of 100-2000 and a relative molecular mass of 242.16. It is a white fibrous or granular powder, odorless, tasteless, and hygroscopic, insoluble in organic solvents.
Styrene-butadiene rubber (SBR): As a rubber-based adhesive, SBR is researched for lithium-ion battery cathode materials due to its excellent flexibility and bonding strength. It can effectively buffer the volume changes of electrode materials during charge-discharge cycles, reducing the shedding of active materials, thereby improving battery cycle stability.
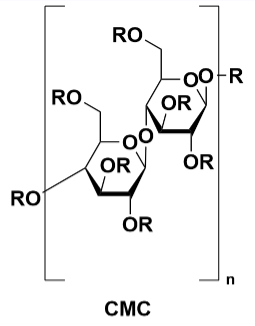
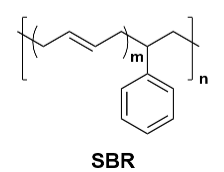
Figure 7 CMC and SBR Structure
Binding mechanism: The groups on the surface of SBR react with the groups on the surface of the copper foil to form chemical bonds. SBR emulsion itself is a balance of hydrophilic and hydrophobic properties, on one hand, it organically combines graphite through hydrophobicity, and on the other hand, the hydrophilic groups react with the groups on the copper foil surface through condensation reaction. CMC-Na acts as a stabilizer and suspension dispersant, assisting in the adhesion of SBR, and ensuring more uniform dispersion of SBR, while the repulsive action of space charges ensures the stability of the entire system.
CMC and SBR in actual lithium battery graphite anodes are complementary and indispensable, which is the result of long-term industrial practice. If only CMC is used as an adhesive, the condition is that the electrode sheet thickness is thin, no rolling process or low compacting density of the electrode sheet. In actual electrode sheets, due to the requirement of energy density, graphite electrode sheets must be rolled and the compacting density is high. In this case, CMC adhesive cannot be used alone because CMC is brittle, and the structure collapses after rolling, causing severe electrode sheet powdering and making it unusable. Additionally, SBR cannot be used alone as an adhesive because it is difficult to prepare slurry, SBR does not have suspension and dispersion functions, slurry will settle, and too much SBR will cause electrode sheet swelling in the electrolyte.
Using CMC and SBR together can basically solve the above-mentioned problems. Graphite materials themselves are not hydrophilic and difficult to disperse in aqueous systems. The role of CMC is to act as a dispersant to disperse graphite and conductive additives. Additionally, CMC forms a gel in water, thickening the slurry. During large-scale coating, the presence of the gel structure retains moisture and stabilizes the slurry, maintaining uniformity over a certain period, facilitating large-scale production. Introducing SBR, as SBR emulsion is water-soluble, SBR itself is a flexible material with good adhesive properties, ensuring the electrode sheet does not powder under high compacting pressure, and the adhesive strength of the rolled electrode sheet is high.
Alginate (ALG): Due to its excellent biocompatibility and gel-forming ability, ALG is commonly used as a natural polymer material in the food industry, pharmaceuticals, and cosmetics. It can quickly form stable gels in water, suitable as a stabilizer or thickener. ALG's biodegradability and renewable sources make it an environmentally friendly material. However, ALG's main drawback is its sensitivity to pH, which may affect its stability in some applications.
Binding mechanism: ALG's binding effect mainly relies on the carboxyl groups on its molecular chain, which can form stable three-dimensional network structures through ion crosslinking. In some cases, ALG can enhance the mechanical properties of its gels by crosslinking with metal ions such as calcium ions. Modified ALG can optimize its binding performance and stability by adjusting its crosslinking degree through chemical or physical methods.
However, ALG's main limitations are its sensitivity to pH, which may affect its stability in some applications. Additionally, the gel strength of ALG may not be as strong as some synthetic polymers, and in some cases, it may be sensitive to electrolytes, which may limit its use in high ionic strength environments.
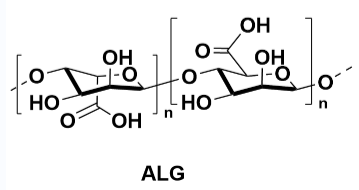
Figure 8 ALG Structure
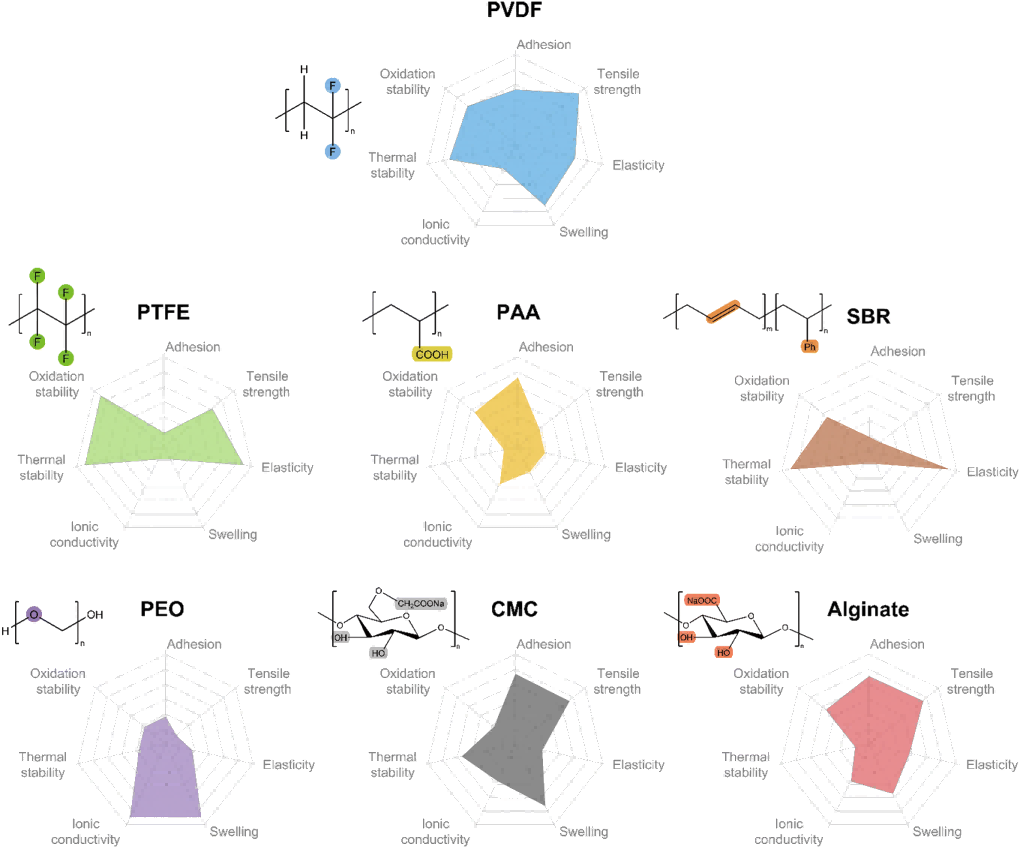
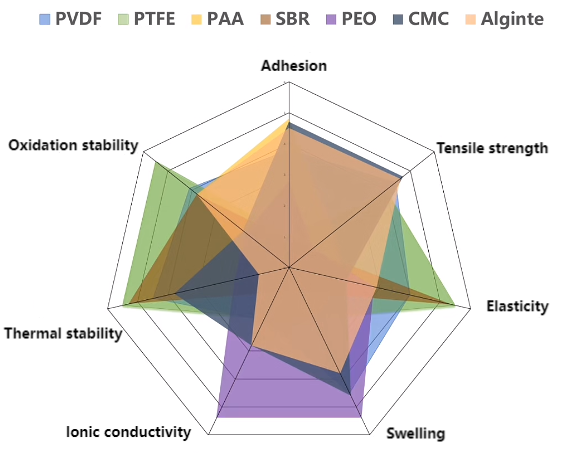
Figure 9 Properties of different binders
· Good adhesive strength and high tensile strength to ensure the integrity of the electrode structure;
· Good flexibility to withstand volume changes and strain variations of the electrode;
· Excellent thermal stability to adapt to a wide temperature range;
· Stable chemical/electrochemical properties to adapt to high voltage windows;
· Good dispersibility to ensure uniform coating of the electrode slurry;
· Sufficient electron/lithium-ion diffusion channels to ensure excellent electrochemical performance;
· Low cost and simple preparation methods to facilitate industrial promotion.




The lab focuses on solid-state battery research to overcome traditional lithium batteries' safety and energy density issues, supporting environmental sustainability. It develops innovative solid-state electrolytes, refines electrode materials, and investigates ion transfer and interface stability to revolutionize battery technology.

The electric vehicle battery industry is rapidly developing, focusing on technological innovation, market competition, and sustainability. Research hotspots include solid-state batteries, new types of electrolytes, BMS optimization, and recycling technologies. The environmental adaptability, safety, and economic viability of batteries are key research areas, and the industry is expected to undergo more innovation and transformation.

Specializing in battery preparation technology research, the focus is on overcoming existing energy storage challenges by innovating in electrode materials, battery chemistry, and manufacturing processes to improve performance, safety, and reduce costs. Sustainability and recycling technologies for batteries are also emphasized to mitigate environmental impacts and foster the growth of green energy.