Lithium-ion batteries have been widely used in consumer electronics, electric vehicles, aerospace and other fields due to their high energy density, high coulomb efficiency, long service life, no memory effect, low self-discharge characteristics and chemical potential of different electrode designs. In recent years, lithium-ion batteries have gradually occupied the main market share of electric vehicles, energy storage systems and mobile electronic devices.
Since the first commercialization of lithium-ion batteries by Sony in 1990, the anode materials have been carbon-based materials, while the cathode materials have made great progress and are the most critical materials to promote the performance of lithium-ion batteries. The particle size, morphology, specific surface area, tap density, structure, composition and other physical and chemical properties and electrochemical properties of lithium ion battery cathode materials have an important influence on the application of lithium ion battery cathode materials. Therefore, this article will introduce the cathode materials of lithium ion batteries.
The ideal cathode material should have the following characteristics:
1.High operating voltage: The Gibbs free energy is large enough during the discharge reaction of the battery, that is, a high working open circuit voltage is generated, thus showing a higher specific capacity;
2.Large theoretical specific capacity: the same mass can accommodate more lithium ions, and can be reversibly embedded and removed; the oxidation state of transition metal ions is variable to ensure the charge balance during the charge-discharge cycle;
3.Long cycle life: the change of material structure should be fully reversible during the insertion/extraction process of lithium ion during charge and discharge, so as to ensure that the material structure is not damaged;
4.Excellent rate performance: high lithium ion diffusion coefficient, high diffusion rate inside and on the surface of the electrode material;
5.Good chemical stability: less chemical reaction with electrolyte in the process of material storage and use;
6.The preparation process is simple and environmentally friendly;
7. Relatively high electronic and ionic conductivity.
At present, the existing cathode materials of lithium-ion batteries are mainly divided into the following categories:
(1) Layered LiMxO2 (M=Co, Ni, Mn) cathode materials and ternary materials;
(2) LiMn2O4 cathode material with spinel structure;
(3) Olivine-structured LiFePO4 cathode materials;
(4) LiMnPO4, LiCoPO4, Li3V2(PO4)3 and other polyanion materials. In addition, there are a relatively small number of multi-phase lithium storage materials and organic cathode materials. The following is a brief introduction to various cathode materials.
1.Layer-structured LiMxO2 (M=Co, Ni, Mn) cathode materials and ternary materials: The lithium cobalt oxide is selected as a typical material for introduction. The lithium cobalt oxide material is a layered structure, and its structure diagram is shown in Figure 1. Lithium cobalt oxide cathode materials are generally used in 3C batteries such as mobile phones and tablets. Because of its high energy density, the battery can be made smaller and has a longer battery life.
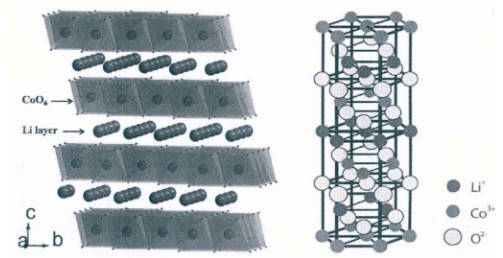
Figure-1-Crystal-structure-diagram-of-LiCoO2
The theoretical specific capacity of lithium cobalt oxide is 274 mAh/g, but the actual capacity cannot reach the theoretical specific capacity. This is because the lithium cobalt oxide cathode material will undergo a phase transition with the de/intercalation of lithium ions during the charge and discharge process. When the lithium is de/intercalated, the lithium cobalt oxide will be unstable, and the oxygen loss reaction will occur which makes the lithium cobalt oxide undergo an irreversible phase transition, from ordered to disordered. Moreover, the hexagonal structure will also be transformed into a monoclinic structure, eventually leading to the failure of the material.
2.Spinel LiMn2O4 cathode material: LiMn2O4 is the spinel phase structure and black gray powder. It has the advantages of low price, high potential, environmental friendliness and high safety performance. The disadvantage is that the energy density is relatively low and the cycle performance is poor. During the repeated charge and discharge process of the battery, the dissolution of manganese will occur, resulting in a decrease in battery performance. Lithium manganate has been applied in many applications with high cost requirements due to its low preparation cost. The structure of lithium manganate is shown in Figure 2.
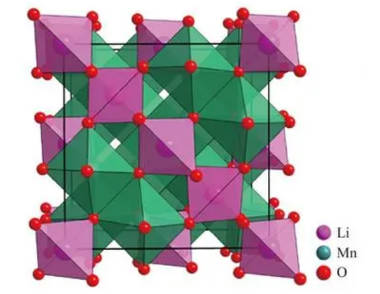
Figure-2-Crystal-structure-diagram-of-LiMn2O4
3.LiFePO4 cathode materials with olivine structure: LiFePO4 is olivine structure. Lithium iron phosphate materials have the advantages of low price, long cycle life, good thermal stability, high safety, and no precious toxic metal elements. The disadvantages are low energy density, low electronic conductivity, and low compaction density. Due to the role of P-O bond, lithium iron phosphate has a stable structure. In the process of deintercalation of lithium ions, it will not have obvious structural damage problems like other cathode materials, and has the characteristics of deep charge and discharge. The structure diagram of lithium iron phosphate is shown in Figure 3.
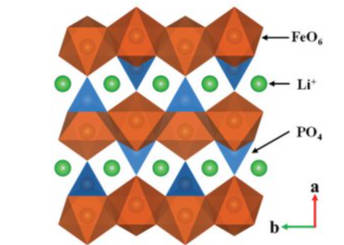
Figure-3-Crystal-structure-diagram-of-LiFePO4
At present, most of the automobile power batteries and energy storage batteries are made of lithium iron phosphate as the cathode material. Lithium iron phosphate cathode material occupies the largest share of the cathode material market due to its high safety, low cost and long cycle.
4.Polyanionic materials such as LiMnPO4, LiCoPO4, Li3V2(PO4)3: Taking lithium manganese phosphate as an example, its chemical formula is LiMnPO4 and it is a natural mineral or synthetic ternary lithium battery electrode material. The material has an olivine-like crystal structure, resulting in stable physical and chemical properties when used as an electrode material. Lithium manganese phosphate has a specific capacity of 171 mAh/g and a discharge platform of about 4.1 V (vs Li+/Li), which also makes lithium manganese phosphate an ideal material for a new generation of lithium-ion power batteries. Its structure diagram is shown in Figure 4.
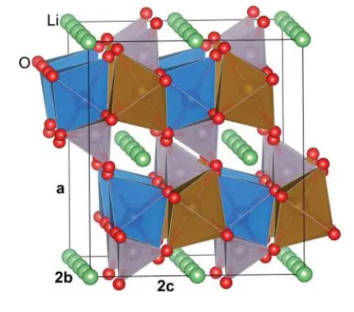
Figure-4-Crystal-structure-diagram-of-LiMnPO4
Although LiMnPO4 has many advantages, the electronic conductivity and lithium ion diffusion performance of LiMnPO4 are seriously restricted by the olivine structure, and it needs to be improved in the future. Figure 5 is the charge-discharge curves of common cathode materials.
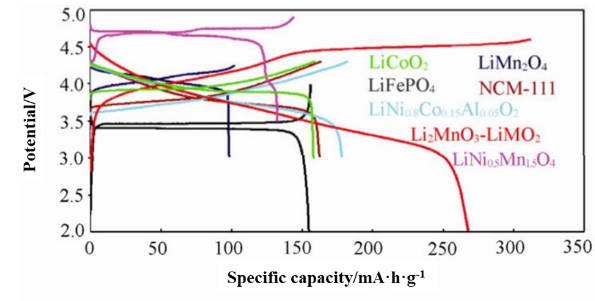
Figure-5-The-charge-and-discharge-curves-of-common-cathode-materials
At present, the research topics of cathode materials of lithium-ion batteries mainly focus on the following aspects:
1.High energy density materials: Researchers are working to develop nickel-rich nickel-cobalt-manganese oxide (NCM) and nickel-cobalt-aluminum oxide (NCA) to improve the energy density of batteries. These materials can provide higher capacity while ensuring cycle life.
2.Solid-state battery materials: Solid-state batteries are regarded as the frontier of next-generation battery technology. Researchers are exploring cathode materials suitable for solid-state batteries, such as lithium-rich or sulfur-containing materials, which can improve the safety and energy density of batteries.
3.The exploration of green and sustainable materials: With the increasing awareness of environmental protection, the research of low cobalt or cobalt-free cathode materials has been paid more and more attention. Lithium iron phosphate and manganese-based materials have attracted attention due to their low cost and environmental friendliness.
4.Nanostructure and composite materials: Through the design of nanocrystallization and composite materials, researchers can improve the conductivity and reaction rate of materials, thereby improving the charge and discharge efficiency and cycle stability of batteries.
5.The research method of combining theory with experiment: Using the method of combining computer simulation with experiment, researchers are deeply understanding the electrochemical behavior of materials to guide the design of new materials.
These research topics not only promote the continuous progress of lithium-ion battery technology, but also lay the foundation for the optimization of future energy storage solutions.




The lab focuses on solid-state battery research to overcome traditional lithium batteries' safety and energy density issues, supporting environmental sustainability. It develops innovative solid-state electrolytes, refines electrode materials, and investigates ion transfer and interface stability to revolutionize battery technology.

The electric vehicle battery industry is rapidly developing, focusing on technological innovation, market competition, and sustainability. Research hotspots include solid-state batteries, new types of electrolytes, BMS optimization, and recycling technologies. The environmental adaptability, safety, and economic viability of batteries are key research areas, and the industry is expected to undergo more innovation and transformation.

Specializing in battery preparation technology research, the focus is on overcoming existing energy storage challenges by innovating in electrode materials, battery chemistry, and manufacturing processes to improve performance, safety, and reduce costs. Sustainability and recycling technologies for batteries are also emphasized to mitigate environmental impacts and foster the growth of green energy.