Electrolyte is the key medium for ion transfer in lithium-ion batteries, mainly composed of the following three parts:
Solvent: As the base medium of the electrolyte, it provides a stable chemical environment that allows lithium ions to move freely within the battery. The chemical and thermal stability of the solvent directly affects the safety and cycle life of the battery.
Lithium salt: Lithium salt is the ion source in the electrolyte, which dissociates lithium ions in the solvent, and these ions realize charge transfer through migration during battery charging and discharging. Without effective lithium salts, the electrolyte will not conduct electricity and the battery will not work properly.
Additives: Additives are used to optimize the performance of the electrolyte, including improving electrical conductivity, thermal and chemical stability, as well as improving the cycle life and safety of the battery. For example, additives can help form a stable electrode/electrolyte interface, reducing side reactions and improving battery efficiency and life.
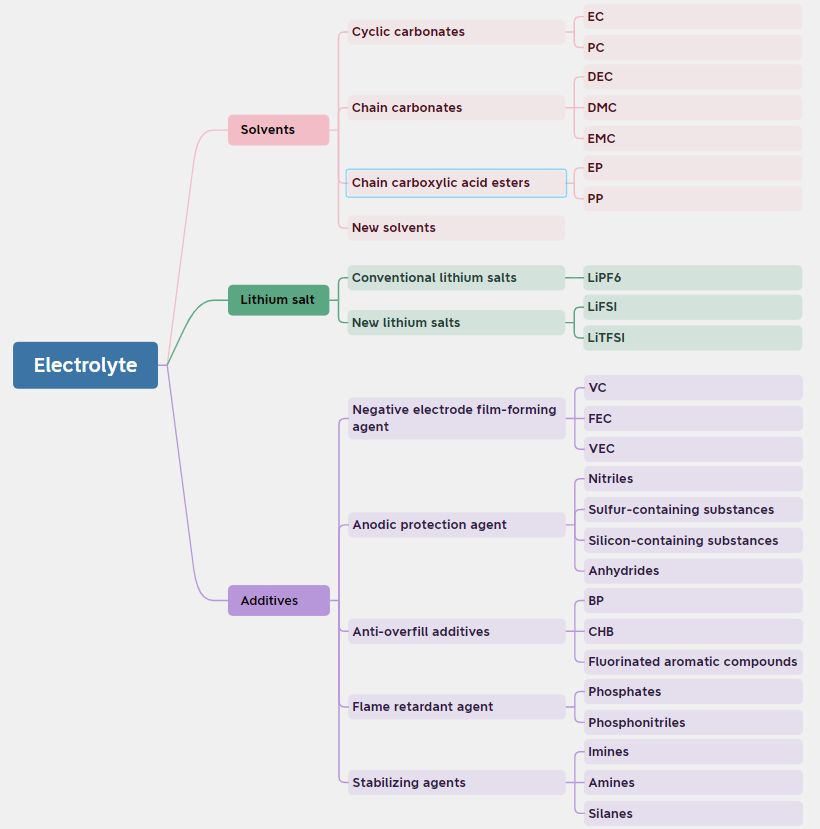
Figure 1 Electrolyte composition
The solvent should exhibit excellent chemical stability, compatibility with the positive material and oxidation resistance, as well as good reduction resistance at the negative electrode to prevent side reactions. In addition, it must be inert to the diaphragm and bonding agent to avoid dissolution and protect the lugs and collector from corrosion. The solvent must also be able to effectively dissolve the lithium salt, which requires it to have the proper polarity, expressed as a suitable dipole moment or dielectric constant. To facilitate lithium ion migration, the electrolyte should have a low viscosity and a liquid temperature range that matches the conditions of battery use. Low vapor pressure helps to reduce solvent loss through evaporation, especially when used in large quantities. Ideally, electrolyte solvents should also be non-toxic and cost-effective for large-scale applications.
Table 1 Introduction of common solvent applications
Solvents | Characteristics | Suitable scenarios | Unsuitable scenarios | Recommended dosage range (% solvent) |
EC | High dielectric constant, good film forming ability, high viscosity | High energy density battery, high temperature environment | Low temperature applications, high voltage applications | Low temperature application: 15~25%, Regular and high temperature: 30~45% |
PC
| Dielectric constant lower than EC, high viscosity, but low freezing point | Soft pack battery, low multiplication, long cycle | High voltage real, high magnification, low temperature, | 3~35%
|
DEC
| Low viscosity and high electrochemical stability, wide liquid range | High temperature, long cycle, conventional | Natural graphite negative electrode | 10~60%
|
DMC | Lower viscosity and good solubility, narrow liquid range | Steel-cased batteries, aluminum-cased batteries, high voltage, high efficiency | High rate, high voltage, low temperature | 10~70% |
EMC | Low viscosity and good solubility with wide liquid range | Most applications, outstanding low temperature performance | / | 10~70%
|
EP | Low viscosity and moderate boiling point | Low temperature, high efficiency, high conductivity, cylindrical cells, aluminum shell batteries | High Temperature, Soft Pack Battery | 5~45% |
*The above is for reference only, please refer to your own experiments.
Lithium salt must firstly have high ionic conductivity to support fast lithium ion migration and high efficiency charging and discharging process. Secondly, they must need to have high electrochemical stability and cannot react with positive and negative electrode materials, diaphragms, binders, collectors, etc. They are mainly resistant to oxidation and reduction. Solubility is also an important factor, with sufficient solubility helping to maintain the desired lithium ion concentration. Special applications may also need to consider special requirements such as high voltage stability or low temperature performance, along with cost effectiveness and environmental impact. Common lithium salt options include LiPF6, LiFSI and LiBOB.
Table 2 Types of organo lithium salts
Organic lithium salt | CAS No. | Molecular weight (g/mol) | Advantages | Disadvantages |
LiBOB | 244761-29-3 | 193.8 | Excellent thermal stability (decomposition temperature 385°C), no generation of HF. | Poor solubility, low conductivity, prone to gas generation |
LiDFOB | 409071-16-5 | 143.8 | Improvement of high and low temperature performance of battery cells | High cost and easy to absorb water |
LiFSI | 171611-11-3 | 187.1 | High conductivity and good thermal stability (decomposition temperature 308°C) | High cost, corrosive collector |
LiTFSI | 90076-65-6 | 287.1 | Good thermal stability (decomposition temperature 337°C) | High cost, corrosive collector |
(1) Anode film-forming additives
In the electrolyte of lithium-ion batteries, anode film-forming additives play a crucial role. By preferentially reducing and decomposing on the anode surface, they promote the generation of a stable SEI film and significantly reduce solvent co-embedding, thus reducing the irreversible capacity loss in the initial cycle. A proper additive can significantly improve battery performance.
Selection strategy:
Reduction Reaction Constant Study: In-depth analysis of the reduction reaction constants of a variety of substances in non-aqueous solvents to screen out candidates that are easy to reduce.
LUMO energy level modulation: the lower the LUMO energy level of a compound, the stronger its reducibility. By introducing double bond, benzene ring, halogen or sulfur substituent groups, the LUMO energy level can be effectively reduced to enhance its reducing tendency in the negative electrode of the battery.
(2) Cathode Additives
Cathode additives for lithium-ion battery electrolytes mainly include nitrile compounds, fluorine-containing compounds, and organic phosphorus compounds. Among them, nitrile compounds are ideal choices for cathode protection due to their wide electrochemical window and excellent oxidative stability. These characteristics impart low flammability under high voltages, providing additional safety assurance for batteries.
Their primary mechanisms of action are as follows:
They form a uniform and dense protective film on the cathode surface, effectively isolating the electrolyte from direct contact with the cathode material.
They prevent the oxidation decomposition of the electrolyte, thereby avoiding corrosion of the cathode material by corrosive substances like HF, while also inhibiting the dissolution of metal ions.
(3) Anti-overcharge additive
In order to prevent the battery from overcharging and improve the safety of use, a variety of anti-overcharging additives have been developed, including redox shuttle type and electro-polymerization type.
①Redox shuttle type additives:
These additives block the current through redox reaction when overcharging, and their oxidation potential is precisely regulated in a range higher than the full charge potential of the positive electrode and lower than the decomposition potential of the electrolyte. When the battery charging voltage exceeds the normal range, the additive oxidizes at the positive electrode, and the oxidation products diffuse to the negative electrode for reduction, forming a shuttle mechanism until the end of overcharging. The energy of overcharging is converted into heat dissipation, which guarantees the safety of the battery.
② Electropolymerization type anti-overcharging additives:
Under overcharging conditions, this type of additive undergoes an electropolymerization reaction on the surface of the positive electrode, forming a protective conductive polymer. The hydrogen gas generated by the reaction may trigger the CID (Current Interrupt Device) action, cutting off the current. The formation of the conductive polymer may penetrate the diaphragm to form a micro-short circuit, or generate a high impedance polymer film, increasing the internal resistance of the battery, forcing the termination of the charging process, effectively preventing the battery from overcharging.
In addition to these additives, lithium-ion battery electrolyte additives also include flame retardant additives (phosphate ester, phosphonitrile), stability additives (imines, amines, silanes), interfacial activity additives and so on. Electrolyte additives are key components to enhance the comprehensive performance of lithium-ion batteries, and they play an indispensable role in the stable operation and high-efficiency conversion of the battery. It is an important driving force to realize the progress of battery technology.
① EC: polar solvent, dissolving lithium salt and film-forming effect, is an essential component.
② DMC: weak polar solvent, low viscosity, conducive to the increase in conductivity, mostly used in multiplication type and the requirement of good wettability of the electrolyte.
③ EMC: easy to decompose into DMC and DEC in small amount, used in aluminum shell battery with EC.
④ DEC: high boiling point, mixed with EMC and PC, mostly used in high temperature electrolyte.
⑤ PC: polar solvent, high boiling point, used in high temperature storage type electrolyte, but poor compatibility with natural graphite.
The passivation layer formed during the first cycle of lithium battery due to the reaction between lithium ion and electrolyte solid-liquid interphase level is called "Solid Electrolyte Interphase (SEI)". Mature SEI membranes are usually composed of inorganic components (e.g., Li2CO3, LiF, Li2O, etc.) and organic components (e.g., ROCO2Li, ROLi, etc.), which provide the electronic insulation and ionic conductivity required for SEI membranes.
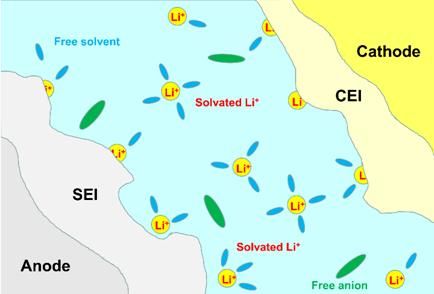
Figure 2 Schematic diagram of the formation of protective films (SEI and CEI) on the surface of the electrode material
Step 1: Nucleation Stage (Nucleation Stage)
Electrolyte decomposition: When the battery is first charged, the Fermi energy level of the negative electrode material (e.g., graphite or silicon) is higher than the lowest unoccupied molecular orbital (LUMO) of the electrolyte component, prompting the transfer of electrons from the negative electrode to the electrolyte, leading to a reduction reaction of the electrolyte on the negative electrode surface.
Initial deposition: The solvent in the electrolyte and the species produced by the decomposition of lithium salts (e.g. RO-, R-, etc., where R stands for alkyl) are deposited on the surface of the negative electrode, forming an initial SEI layer.
Unevenness: Due to the unevenness of the electrolyte decomposition reaction, the initially deposited SEI layer may be thinner in some areas and thicker in others.
Step 2: Growth Stage (Growth Stage)
Continuous Deposition: As the charging process continues, more electrolyte decomposition products are deposited on the negative electrode surface, gradually thickening the SEI layer.
Stabilization: The thickening of the SEI layer helps to stabilize the electrode/electrolyte interface, reducing further decomposition of the electrolyte and thus improving the cycling stability of the battery.
Formation of stable SEI film: Eventually, a stable SEI film that is electrically insulating but ionically conductive is formed, which prevents solvent molecules in the electrolyte from further reacting with the negative electrode while allowing lithium ions to pass through.
Electronic insulator, excellent conductor of Li+;
Broadened electrochemical stabilization window;
Prevention of solvent co-embedding and HF corrosion of electrode materials;
Good self-stability to overcome the negative electrode expansion problem.
The composition and properties of the electrolyte directly affect the formation and properties of the SEI film. For example, the chemical stability of the solvent in the electrolyte, the type and concentration of additives, and the selection of lithium salts all have an impact on the composition, structure and stability of the SEI membrane. Therefore, by optimizing the formulation of the electrolyte, the formation process of SEI film can be regulated to achieve the improvement of battery performance.




The lab focuses on solid-state battery research to overcome traditional lithium batteries' safety and energy density issues, supporting environmental sustainability. It develops innovative solid-state electrolytes, refines electrode materials, and investigates ion transfer and interface stability to revolutionize battery technology.

The electric vehicle battery industry is rapidly developing, focusing on technological innovation, market competition, and sustainability. Research hotspots include solid-state batteries, new types of electrolytes, BMS optimization, and recycling technologies. The environmental adaptability, safety, and economic viability of batteries are key research areas, and the industry is expected to undergo more innovation and transformation.

Specializing in battery preparation technology research, the focus is on overcoming existing energy storage challenges by innovating in electrode materials, battery chemistry, and manufacturing processes to improve performance, safety, and reduce costs. Sustainability and recycling technologies for batteries are also emphasized to mitigate environmental impacts and foster the growth of green energy.