As a core component of modern portable electronic devices, electric vehicles and energy storage systems, the performance of lithium-ion batteries directly affects the efficiency and lifetime of these devices. Compaction density is a key parameter in the design of lithium-ion battery cells, which has a significant impact on the energy density, power performance and cycle life of the battery. In this paper, we will discuss in detail the impact of optimal compaction density on the design of lithium-ion battery cells and analyze the electrochemical and structural design of the cells in depth. This paper aims to provide battery design engineers and researchers with comprehensive theoretical guidance and practical reference to promote the development of lithium-ion battery technology.
Compaction density refers to the bulk density of the electrode material after compaction under a certain pressure, usually expressed in g/cm³. Optimization of the compaction density is critical to the performance of lithium-ion batteries. Generally speaking, within the allowable compaction range of the material, higher compaction density can increase the active material content per unit volume, thus increasing the energy density of the battery. At the same time, a suitable compaction density can optimize the pore structure of the electrode, which helps to reduce the volume change of the electrode material during the cycling process, improve the transfer efficiency of ions and electrons, and enhance the battery's cycle multiplier performance.
Figure 1 Schematic diagram of roller press production
Factors affecting the compaction density mainly include the nature of the electrode material, preparation process and compaction conditions.
● Electrode material properties: including the particle size distribution, morphology and specific surface area of the material.
● Preparation process: including slurry mixing, coating and drying process.
● Compaction conditions: including compaction pressure, temperature and time, etc.
Compaction density directly affects the pore structure of the electrode material and the ion transport path, thus affecting the kinetic performance of the electrochemical reaction.
◆ Ion transport: moderate compaction density can provide a good pore structure to ensure adequate wetting of the electrolyte and rapid ion transport, optimizing the kinetic performance of the electrochemical reaction.
◆ Electron transfer: High compaction density can improve the conductivity of the electrode and enhance the electron transfer efficiency, which is conducive to the electrochemical reaction.
◆ Concentration polarization: moderate compaction density can optimize the pore structure of the electrode, reduce the concentration polarization phenomenon, improve the electrochemical performance of the battery.
◆ Resistive polarization: high compaction density can reduce the internal resistance of the electrode, reduce the resistive polarization phenomenon, and enhance the power performance of the battery.
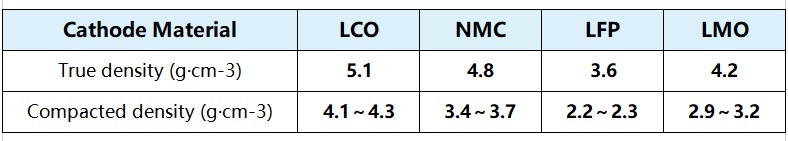
Figure 2 True density of commonly used materials
The overpressure of the pole piece will cause the battery capacity to be reduced, the cycle to deteriorate, and the internal resistance to increase. Pole piece overpressure will make the spherical ternary material large area broken, the newly generated surface has a lot of primary small particles detached from the secondary ball, they either fall from the pole piece because of the lack of contact with the PVDF, or because of the lack of contact with the conductive agent to make the conductivity of the pole piece of the local deterioration of the conductive properties. The creation of new surfaces also increases the specific surface, the contact surface with the electrolyte, and the side reactions, which results in reduced battery performance, such as battery gassing and cycle decay. Overpressure can also cause deformation of aluminum foil, brittle pole piece, easy to break, and increase the internal resistance of the battery.
Porosity of the pole piece refers to the volume of the internal pores of the pole piece after rolling as a percentage of the total volume of the pole piece after rolling. Too low a porosity will reduce the electrolyte volume to the electrode infiltration rate, affecting the performance of the battery; too high will reduce the energy density of the battery, wasting the effective space. Porosity testing can be done by mercuric pressure, nitrogen adsorption, liquid absorption, estimation, etc. Mercuric pressure is the commonly used method. The specific operation steps of the liquid absorption method are as follows: cut an appropriate amount of pole piece and measure the mass m of said pole piece; measure the volume V of said pole piece; place said pole piece into a container, said container is set with electrolyte or other solvents (the density of solvents is ρ), completely immerse said pole piece and immerse it for a certain period of time; take out said pole piece, place it on a filter paper, suction wipe it to a constant weight, and measure the mass m1 of said pole piece; Calculate the porosity ε of the pole piece according to the formula ε =(m1-m)/ρV×100%.The estimation method is relatively simple, and the porosity of the pole piece can be estimated based on the difference between the true density of the material and the compacted density of the pole piece. The equation for calculating the porosity of the pole piece is as follows:
Pole piece porosity (%) = (true density of mixture - compacted density of pole piece) / true density of mixture × 100%.
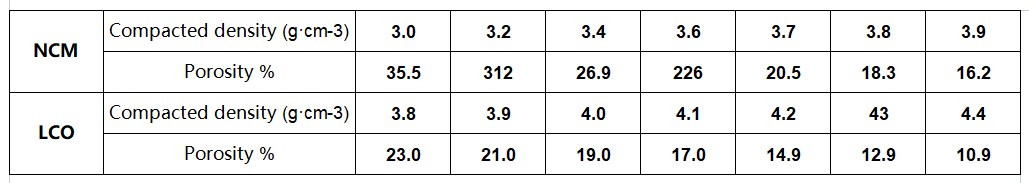
Figure 3 Compaction density and porosity of commonly used materials
The nature of the electrode material has an important influence on the compaction density. By optimizing the properties of electrode materials, the compaction density and battery performance can be improved.
▲ Particle size distribution: Controlling the particle size distribution of the electrode material can improve the compaction density and electrical conductivity of the material. Using a mixture of nanoparticles and micron particles, the packing density and electrochemical properties of the material can be optimized. The use of multistage particle size distribution, i.e., mixing particles of different sizes in the electrode material, can improve the filling density of the material. Smaller particles can fill the voids between larger particles, improving the overall compacted density and electrical conductivity.
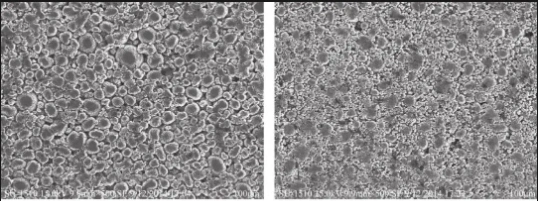
Figure 4 SEM images of cathode electrodes with different particle size distributions
▲ Morphology: Controlling the morphology of the electrode material can improve the compaction density and electrochemical properties of the material. Spherical particles have a high packing density and can improve the compaction density of the electrode. Spherical particles can also reduce the expansion and contraction of the electrode during cycling and improve the cycling stability of the battery. Flaky particles have a large specific surface area, which helps to improve the activity and reaction rate of the electrode material. By optimizing the thickness and width of the flake particles, the compaction density and electrochemical performance of the electrode can be improved. Using a mixture of spherical particles and flake particles, the packing density and electrical conductivity of the material can be optimized.
▲ Specific surface area: Controlling the specific surface area of the electrode material can improve the compaction density and electrochemical properties of the material. High specific surface area materials can increase the contact area between the electrode and the electrolyte and improve the power performance of the battery. However, high specific surface area materials usually have low compaction density, so it is necessary to find a balance between specific surface area and compaction density.
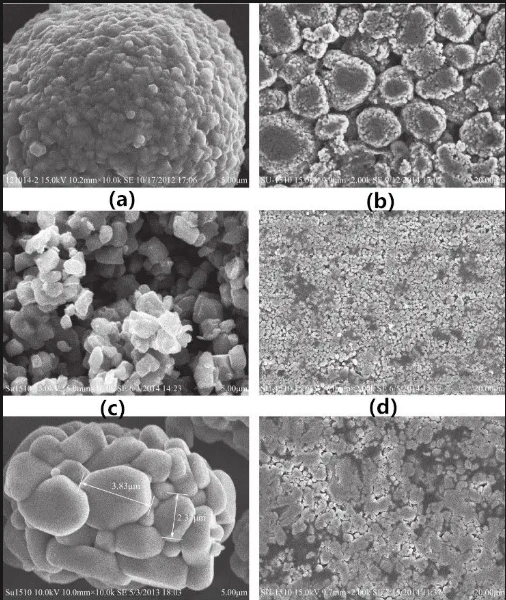
Figure 5 SEM images of ternary materials with different morphologies and their electrodes
The compaction density of the electrodes can be significantly improved by optimizing the paste preparation, coating process and drying process.
● Slurry preparation: Controlling the formulation and mixing process of the slurry can improve the compaction density and electrochemical properties of the electrode. The formulation of the slurry has a direct impact on the compaction density and electrode performance. Reasonable selection and optimization of the ratio of conductive agent, binder and solvent can improve the uniformity and coating quality of the slurry. An efficient mixing process can ensure the homogeneity of the slurry and reduce bubbles and particle agglomeration. Commonly used mixing equipment includes planetary mixers and high-speed shear mixers. Optimizing the ratio of conductive agent and binder can improve the uniformity and coating quality of the slurry.
● Coating process: controlling the parameters of the coating process can improve the compaction density and electrochemical properties of the electrode. Controlling the coating speed can improve the uniformity and quality of the coating. Too fast a coating speed may result in an uneven coating, while too slow a coating speed may affect productivity. Coating thickness has a significant effect on the compaction density and electrochemical performance of the electrode. Optimizing the thickness and uniformity of the coating can improve the electrochemical performance and mechanical strength of the electrode.
● Drying process: Controlling the parameters of the drying process can improve the compaction density and electrochemical properties of the electrode. Optimizing the temperature and time of drying can improve the mechanical strength and electrical conductivity of the coating.
By optimizing the compaction pressure, compaction temperature and compaction time, the compaction density and electrochemical properties of the electrode can be improved.
◆ Compaction pressure: Controlling the compaction pressure can improve the compaction density and electrochemical properties of the electrode. Appropriately increasing the compaction pressure can improve the packing density and electrical conductivity of the material, but too high compaction pressure may lead to increased mechanical stress of the material, increasing the risk of cracking and failure of the material. The multi-stage compaction process can gradually increase the pressure, reduce the stress concentration phenomenon of the material, and improve the mechanical strength and stability of the electrode.
◆ Compaction temperature: controlling the compaction temperature can improve the compaction density and electrochemical properties of the electrode. Appropriately increasing the compaction temperature can improve the plasticity and electrical conductivity of the material, but too high a compaction temperature may lead to thermal decomposition and performance degradation of the material. And the use of hot compaction process can significantly improve the compaction density and mechanical strength of the electrode under the appropriate temperature and pressure.
◆ Compaction time: controlling the compaction time can improve the compaction density and electrochemical properties of the electrode. Appropriate extension of the compaction time can improve the packing density and electrical conductivity of the material, but too long compaction time may lead to material fatigue and performance degradation.
Different electrode materials have different physicochemical properties, and their suitable compaction densities are also different. Positive electrode materials such as lithium cobalt oxide (LiCoO₂), nickel cobalt manganese oxide (NCM), and lithium iron phosphate (LiFePO₄) have different densities and pore structures, and have different requirements for compaction density. The optimization of the compaction density of anode materials such as graphite and silicon-carbon composites also needs to take into account the expansion characteristics and cycling stability of the materials.
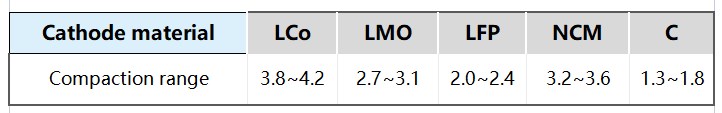
Figure 6 Compaction design range for commonly used material systems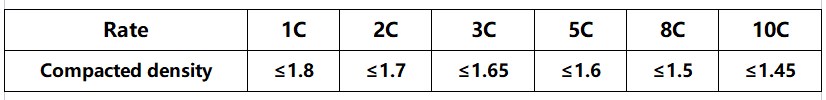
Figure 7 Reference for graphite compaction density design
The permeability and ionic conductivity of the electrolyte are closely related to the compaction density of the electrode. High compaction density electrodes require the selection of a highly permeable electrolyte to ensure that the electrolyte can fully infiltrate the electrode material and improve the ion transfer efficiency. Solvents used include ethylene carbonate (EC), dimethyl carbonate (DMC) and acetonitrile (ACN). The selection of lithium salts requires a combination of solubility, conductivity and thermal stability. Commonly used lithium salts include lithium hexafluorophosphate (LiPF₆), lithium tetrafluoroborate (LiBF₄), and lithium bis(trifluorosulfonyl)imide (LiTFSI). Additives include lithium fluoride (LiF), sulfuryl fluoride (SO₂F₂), and ionic liquids.
▲ Electrode thickness: Electrode thickness and compaction density together affect the energy density and power performance of the battery. Optimizing the electrode thickness can ensure high energy density while ensuring good ion transport and mechanical strength.
▲ Collector design: The selection and design of the collector has a significant impact on the conductivity and mechanical strength of the electrode. Aluminum and copper foils are commonly used collector materials, and by optimizing their thickness and surface treatment, the conductivity and adhesion of the electrode can be improved.
The aperture and thickness of the diaphragm have an important influence on the wetting of the electrolyte and ion transport. The safety and cycle life of the battery can be improved by optimizing the material and structure of the diaphragm. The selection of diaphragm materials requires comprehensive consideration of their mechanical strength, thermal stability and ionic conductivity. Commonly used diaphragm materials include polyethylene (PE), polypropylene (PP) and composite diaphragms (PE/PP/PE). The porosity of the diaphragm directly affects the wetting of the electrolyte and ionic transport properties. Moderate porosity ensures good electrolyte wetting and ionic transport, optimizing the electrochemical performance of the battery. The thickness of the diaphragm needs to be minimized under the premise of ensuring mechanical strength to reduce the internal resistance of the battery and improve the power performance. Usually, the thickness of the diaphragm for lithium-ion batteries is between 20 μm and 30 μm.
The assembly process of the battery cell has an important impact on the compaction density and battery performance. The optimization of the structure of the battery cell includes the arrangement of the pole pieces, the design of the pole lugs, and the shell encapsulation. The arrangement of pole lugs needs to take into account factors such as energy density, power performance and thermal management of the battery. Commonly used pole arrangement methods include winding and stacking. The design of lugs needs to ensure good current conduction and thermal management performance. The material of the lugs is usually copper or aluminum foil, and the welding quality of the lugs directly affects the internal resistance and safety of the battery. Case encapsulation requires comprehensive consideration of the mechanical strength, sealing and heat dissipation of the battery. Commonly used case materials include aluminum alloy and stainless steel, and the encapsulation method usually adopts laser welding or ultrasonic welding. Cell structure optimization can improve cell consistency and production efficiency.
The optimal compaction density has an important impact on the design of lithium-ion battery cells, which directly determines the energy density, power performance and cycle life of the battery. By systematically understanding the basic concepts and influencing factors of compaction density and optimizing the electrode materials, preparation process and compaction process, the overall performance of the battery can be significantly improved. It is hoped that this paper can provide valuable references for battery design engineers and researchers to promote the development and application of lithium-ion battery technology.




The lab focuses on solid-state battery research to overcome traditional lithium batteries' safety and energy density issues, supporting environmental sustainability. It develops innovative solid-state electrolytes, refines electrode materials, and investigates ion transfer and interface stability to revolutionize battery technology.

The electric vehicle battery industry is rapidly developing, focusing on technological innovation, market competition, and sustainability. Research hotspots include solid-state batteries, new types of electrolytes, BMS optimization, and recycling technologies. The environmental adaptability, safety, and economic viability of batteries are key research areas, and the industry is expected to undergo more innovation and transformation.

We specialize in battery preparation technology research, focusing on overcoming existing energy storage challenges by innovating in electrode materials, battery chemistry, and manufacturing processes to improve performance, enhance safety, and reduce costs. Sustainability and recycling technologies for batteries are also emphasized to mitigate environmental impacts and foster the growth of green energy.