As a high energy density and long-life energy storage device, lithium-ion batteries are widely used in various electronic products, electric vehicles and energy storage systems. However, the performance of lithium-ion batteries decreases with the increase of usage time, and their safety issues become more and more obvious. Therefore, it is of great significance to study the composition and internal reaction processes of lithium-ion batteries to improve their performance and ensure their safety.
Mass spectrometry, as a highly accurate and sensitive analytical tool, can play an important role in lithium-ion battery testing. In recent years, with the development of technology and the emergence of time of flight mass spectrometry (time of flight), gas or liquid chromatography - mass spectrometry (GC-MS/LC-MS), ion trap mass spectrometry (ITMS), inductively coupled plasma mass spectrometry (ICP-MS), time-of-flight secondary ion mass spectrometry (TOF-SIMS) and so on. Mass spectrometry is based on the moving gaseous ions with different sizes of mass-to-charge ratios to achieve the separation, which can realize the qualitative and quantitative analysis of substances In this paper, we will introduce in detail the application of mass spectrometry in lithium-ion battery testing, including its basic principles, its application in different testing sessions and its contribution to the study of battery performance.
A Mass Spectrometer (MS) is an instrument that analyzes the composition of a sample by measuring the ion mass-to-charge ratio (m/z). It works by ionizing the sample, then separating ions with different mass-to-charge ratios by an electric or magnetic field, and then measuring the number of ions by a detector to obtain a mass spectrum of the sample.
Figure 1 shows the Master320, a mass spectrometer sold by Sunway Research and Selection Mall, the mass spectrometer consists of the following main components:
● Ion sources: Ionize sample molecules to generate gas-phase ions. Common ion sources include electron ionization (EI), chemical ionization (CI), electrospray ionization (ESI), and matrix-assisted laser desorption ionization (MALDI).
● Mass analyzers: Separation of ions with different mass-to-charge ratios by means of an electric or magnetic field. Common mass analyzers include quadrupoles, time-of-flight (TOF), magnetic mass spectrometers, and ion traps.
● Detectors: Detect ions passing through the mass analyzer and convert them into an electrical signal. Common detectors include multipliers and photomultiplier tubes.
● Data analysis system: Processes and analyzes detector signals, generates mass spectra, and resolves sample composition and structure.

Figure 1 Mass Spectrometer Master320
Mass spectrometry can be used to analyze the composition of lithium-ion battery electrolytes to determine the types of solvents and salts in the electrolyte and their concentrations. This is important for electrolyte optimization and performance improvement.
Commonly used electrolyte composition: Commonly used lithium ion battery electrolyte consists of solvents (e.g. ethylene carbonate EC, dimethyl carbonate DMC, etc.) and lithium salts (e.g. lithium hexafluorophosphate LiPF6). A mass spectrometer can analyze the ratio and purity of these components.
During the charging and discharging process of a battery, the electrolyte may decompose to produce by-products that may affect the performance and lifetime of the battery. Mass spectrometry can be used to detect and analyze these decomposition products and assess their impact on the battery.
Electrolyte Decomposition Products: Common electrolyte decomposition products include fluorides, phosphates and organic compounds. Mass spectrometry can detect the types and concentrations of these products and assess their impact on battery performance.
Mass spectrometry can be used to analyze the composition and structure of cathode materials for lithium-ion batteries and to evaluate the performance and stability of cathode materials.
Commonly used cathode materials: Commonly used cathode materials for lithium-ion batteries include lithium cobaltate (LiCoO2), lithium nickel cobalt manganese (NCM), and lithium iron phosphate (LiFePO4). Mass spectrometers can analyze the elemental composition and structure of these materials and evaluate their performance.
Mass spectrometry can be used to analyze the composition and structure of anode materials for lithium-ion batteries and to evaluate the performance and stability of anode materials.
Commonly used anode materials: Commonly used anode materials for lithium-ion batteries include graphite, silicon-carbon composites and lithium titanate (Li4Ti5O12). Mass spectrometers can analyze the elemental composition and structure of these materials and evaluate their performance.
The solid electrolyte interface (SEI) membrane is a protective film on the anode surface of lithium-ion batteries, which has an important impact on the cycle life and safety of the battery. Mass spectrometry can analyze the composition and structure of the SEI membrane and evaluate its impact on battery performance.
SEI membrane composition analysis: Mass spectrometry can detect elements and compounds in SEI membranes, determine their chemical composition and structure, and evaluate their stability and protection performance. As shown in Fig. 2, S (m/z=31.97), SO (m/z=47.97), SO2 (m/z=63.96) and SO3 (m/z=79.96) related chromatographic peaks were detected on the negative graphite surface using TOF-SIMS, in which the peaks corresponding to mass-to-charge ratios of 47.97 and 63.96 were the fragmentation ions peaks of SO3, whereas the peaks corresponding to SO4 (m/z=95.95) and SO3 (m/z=95.95) were not detected. SO4 (m/z=95.95), indicating that the SEI films generated on the graphite surface are composed of sulfites, such as the inorganic salt Li2SO3 and the organic film ROSO2Li. On the contrary, no peaks with mass-to-charge ratios corresponding to SO (m/z=47.97), SO2 (m/z=63.96), SO3 (m/z=79.96), and SO4 (m/z=95.95) appeared on the surface of LiCoO2, but ionic peaks attributed to PO2 (m/z=62.96), and PO3 (m/z=78.96) were detected, which indicates that the SEI membranes generated on the surface of LiCoO2 are composed of sulfites, such as the organic salt Li2SO3 and organic membrane ROSO2Li. The substance generated by LiCoO2 is a phosphorus compound, in which the POx fragments may originate from LiPOxFy, such as LiPO2F2 or LiPOF3. In addition, the researchers also detected ion peaks on the anode surface attributed to S (m/z=31.97), which is speculated to originate from the formation of alkyl sulfides on the surface of LiCoO2 [1].
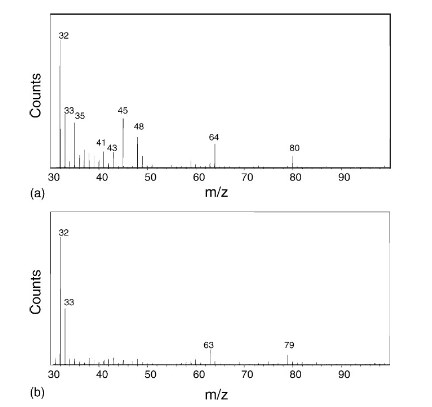
Fig. 2 TOF-SIMS SEI composition analysis of positive and negative electrode surfaces based on LiPF6/PC/ES system (a) Graphite negative electrode (b) LiCoO2/1M LiPF6/PC+5%ES[1]
In the above SEI membrane formation mechanism, we found that lithium-ion batteries in the SEI membrane formation process is often accompanied by the gas production process, in fact, the battery in the formation, high temperature, over-discharge and other conditions may be caused by the battery gas production, these gases in the accumulation of the battery casing lead to increased internal pressure, active substance separation, and even bring potential safety hazards. The components of lithium battery gas production contain H2, CO, CO2, C2H2, CH4, etc. Accurate analysis of the gas composition is conducive to the study of the mechanism of battery flatulence, in order to improve safety performance.
As shown in Fig. 3, under overcharging or overdischarging conditions, the same is accompanied by the generation of gases. In LiCoO2/C system, in the normal voltage range (4.2V-2.5V) charging and discharging cycle, the gas generated contains CO2, CH4, C2H6, etc. In the over-discharge condition, the CO2 content generated in the system is greatly increased, and contains some CO. Under the condition of low potential, the dissolution of the copper foil occurs in the negative electrode and dissolves out of the positive electrode, which leads to the Li+ can't be embedded back to the positive electrode material. At the same time, the degradation of the electrolyte in the system accelerates to produce hydrocarbons; under overcharging conditions, the system also produces a large amount of CO2, and the O2 produced by the decomposition of CoO2 under overcharging conditions further accelerates the degradation of the electrolyte[2].
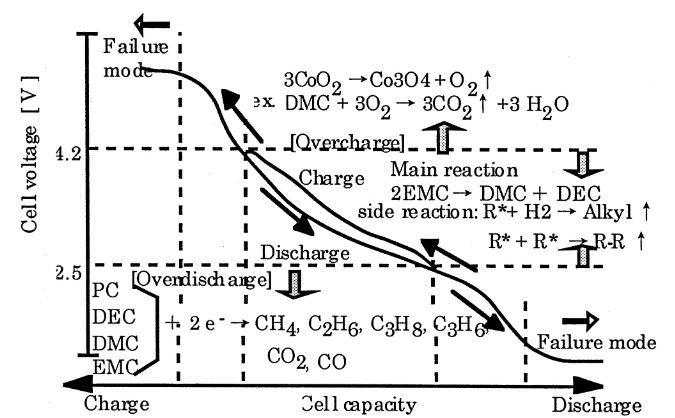
Fig. 3 Diagram of gas production pattern under overfilling and overdischarging conditions[2]
Mass spectrometry can be used to analyze the aging of batteries during use and to assess their life and causes of failure.
Battery aging is mainly due to the deterioration of the electrode materials and the decomposition of the electrolyte. Heating of the electrolyte also leads to the decomposition and further reaction of the main solvent, for example, in the DMC/EC/LiPF6 system at 200 °C, the decarboxylation reaction occurs in the system to generate CO2 and dimethyl ether, etc., and at the same time, the presence of a small amount of water causes the decomposition of lithium salt to generate POF3, and the products further react to generate CH3F and organofluorine-phosphorus compounds[3]. When CH3OLi or CH3CO2Li is added to the above system, the content of CO2, dimethyl ether and CH3F increases, and LiF is generated, and the electrons or lithium ions in the system react with carbonate esters such as DMC to generate corresponding lithium salts and reactive free radicals such as methyl -CH3, and further react to generate gases such as C2H6. In addition, under the multiple effects of electrons, lithium ions and ester exchange reactions, DMC and EC will generate EMC, DEC, etc. in the system[3], and the phenomenon of generating new carbonate esters due to such effects as ester exchange still exists in the recycled LP30+LiC6 system. The degradation reaction roadmap of the DMC/EC/LiPF6 system shown in Fig. 3 can help us better understand the aging mechanism of the electrolyte and is of great significance for the formation mechanism and composition analysis of SEI.
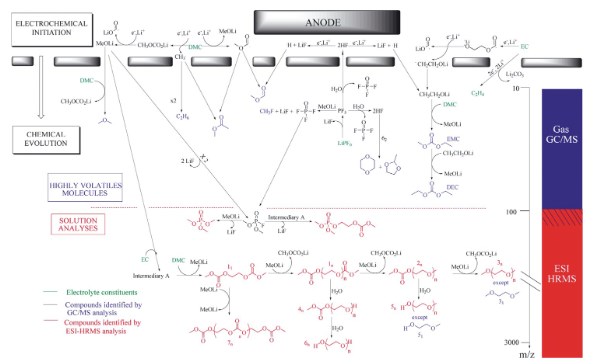
Fig. 4 Overview of DMC/EC/LiPF6 degradation production[3]
Mass spectrometry can be used to analyze battery failures during use and determine the cause and mechanism of the failure. Battery failures may be due to internal short circuits, overcharging and overdischarging, and mechanical damage. Mass spectrometers can detect reaction products and degradation products in faulty batteries and analyze the specific cause of the failure.
Mass spectrometers with high sensitivity and high resolution can accurately detect trace components and subtle changes in samples. This is important for the fine analysis of lithium-ion battery materials and reaction products.
● High sensitivity: The mass spectrometer can detect the content of trace components in a sample and assess their impact on cell performance.
● High resolution: The mass spectrometer resolves subtle changes in the sample, providing high-resolution compositional and structural information to assess the homogeneity and consistency of the cell material.
Mass spectrometry allows for rapid, real-time sample analysis, avoiding the destruction of samples by traditional chemical analysis methods. This is important for real-time testing and performance optimization of battery materials:
● Rapid analysis: The mass spectrometer can analyze the composition and structure of samples in a short period of time, improving testing efficiency.
● Real-time analysis: The mass spectrometer can detect the product changes during the cell reaction in real time, providing real-time analysis of the reaction mechanism.
Mass spectrometers can be used in combination with other analytical techniques (e.g., gas chromatography, liquid chromatography, etc.) to provide a versatile and comprehensive analytical platform. This is of great significance for the comprehensive study of lithium-ion battery materials and reaction mechanisms:
● Multi-functional combinations: Mass spectrometers can be used in combination with gas chromatography, liquid chromatography and other techniques to provide comprehensive analysis of composition and structure.
● Comprehensive analysis platform: The mass spectrometer can perform multiple analyses on the same platform, providing comprehensive material and reaction information and facilitating the overall optimization of cell performance.
While mass spectrometry offers many advantages in lithium-ion battery testing, it also faces a number of challenges:
● Analysis of complex samples: Lithium-ion battery materials and reaction products are complex, and mass spectrometers may face problems of signal overlap and interference when analyzing complex samples.
● Detection of trace components: The content of certain trace components in lithium-ion batteries is low, and the mass spectrometer needs to improve sensitivity and resolution when detecting trace components.
As a high-precision and high-sensitivity analytical tool, mass spectrometer plays an important role in lithium-ion battery testing and research. Through the application of mass spectrometer, the composition and structure of battery materials can be analyzed, the product changes during the battery reaction process can be detected, the performance and stability of the battery can be evaluated, and the scientific basis for the development and optimization of the battery can be provided. In the future, with the continuous progress of technology, mass spectrometer will play a greater role in lithium-ion battery testing and promote the development and application of battery technology.
[1] Ota H, Akai T, Namita H, et al. XAFS and TOF-SIMS analysis of SEI layers on electrodes[J]. Journal of Power Sources, 2003, s119-121(119):567-571.
[2] Kumai K, Miyashiro H, Kobayashi Y, et al. Gas generation mechanism due to electrolyte decomposition in commercial lithium-ion cell[J]. Journal of Power Sources, 1999, s 81-82(9):715-719.
[3] Gachot G, Ribière P, Mathiron D, et al. Gas Chromatography/Mass Spectrometry As a Suitable Tool for the Li-Ion Battery Electrolyte Degradation Mechanisms Study[J]. Analytical Chemistry, 2011, 83(2):478-485.




Technology
December 04, 2025

The lab focuses on solid-state battery research to overcome traditional lithium batteries' safety and energy density issues, supporting environmental sustainability. It develops innovative solid-state electrolytes, refines electrode materials, and investigates ion transfer and interface stability to revolutionize battery technology.

The electric vehicle battery industry is rapidly developing, focusing on technological innovation, market competition, and sustainability. Research hotspots include solid-state batteries, new types of electrolytes, BMS optimization, and recycling technologies. The environmental adaptability, safety, and economic viability of batteries are key research areas, and the industry is expected to undergo more innovation and transformation.

We specialize in battery preparation technology research, focusing on overcoming existing energy storage challenges by innovating in electrode materials, battery chemistry, and manufacturing processes to improve performance, enhance safety, and reduce costs. Sustainability and recycling technologies for batteries are also emphasized to mitigate environmental impacts and foster the growth of green energy.