The phosphorus anode is considered a promising energy storage material due to its high theoretical specific capacity and safe lithium intercalation potential. However, the lithiation/delithiation reactions of multiphase phosphorus and the soluble intermediate products can lead to sluggish reaction kinetics and loss of active materials, affecting the energy density and cycling lifespan of batteries.
A research team led by Dr. Jie Sun from Tianjin University proposed a localized electric field (LEF) strategy to suppress the dissolution of lithium phosphorus compounds (LiPPs) and promote reaction kinetics by optimizing ion-covalent organic frameworks (iCOFs). The LEF induced by imbalanced charge distribution significantly enhances atomic-level charge transfer, enabling rapid electrochemical reactions. Moreover, the team explored two iCOFs with opposite charges, namely TpEB-FSI with a cationic framework and TpPa-SO3Li with an anionic framework, to construct different LEF effects for the phosphorus anode. This effectively addresses the loss of active materials and improves the utilization of the phosphorus anode. The work was published in the top-tier journal Advanced Materials, with Yuc Cao as the first author of the paper.
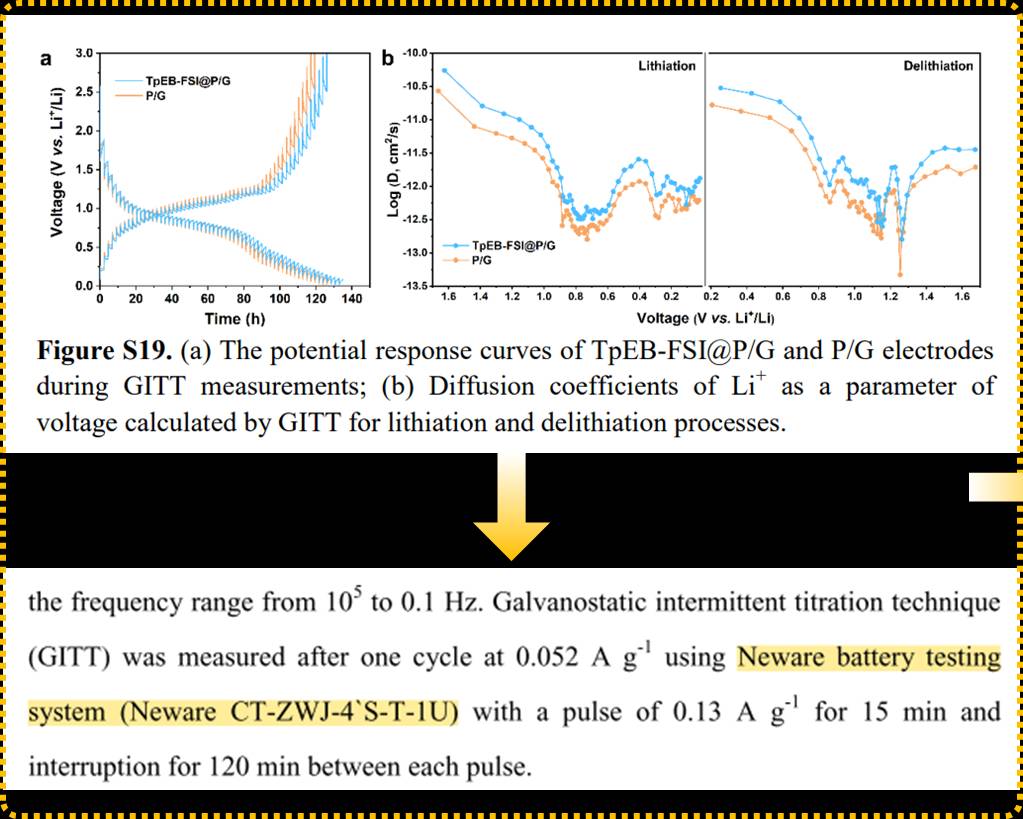
In this study, the galvanostatic intermittent titration technique (GITT) tests were conducted using the CT-ZWJ-4’S-T-1U potentiostat from NEWARE. This potentiostat, when paired with the NEWARE 4-series charge/discharge equipment, allows for data acquisition, testing process monitoring, and more. Additionally, the equipment features an advanced central processing unit capable of parallel data processing and high-speed data transmission, efficiently handling large-scale data and complex computational tasks, making it an excellent choice for battery testing.

Potassium metal anodes are ideal electrode materials for high-energy-density batteries. However, these materials exhibit high reactivity and easily form an unstable solid-electrolyte interface (SEI) upon contact with the electrolyte. This leads to uncontrolled electrodeposition/stripping processes during charge-discharge cycles, resulting in the growth of metal dendrites and reducing the Coulombic efficiency and cycling lifespan of the battery.
Inspired by human skin, a research team led by Prof. Bingan Lu from Hunan University and Prof. Chengxin Wang from Sun Yat-sen University has proposed a novel approach to protect metal interfaces using a metal electrode skin (MES). The MES is composed of a fluorine-doped graphene oxide artificial membrane as the primary protective layer. At the atomic level, the deposition of potassium further promotes the breaking of carbon-fluorine bonds, releasing fluorine that enhances the formation of SEI in situ, acting as a secondary protection layer for the metal anode. The combined effect of this dual protection system stabilizes the metal electrode interface and suppresses dendrite formation. By employing this electrode skin protection, symmetric cells demonstrated a cycle life of up to 2,300 hours, and K@MES||Prussian blue-based cells exhibited over 5,000 cycles. This research strategy provides a new approach for designing and fabricating biomimetic metal electrode interfaces. The work was published in the top-tier journal Nature Communications, with Hongbo Ding as the first author of the paper.
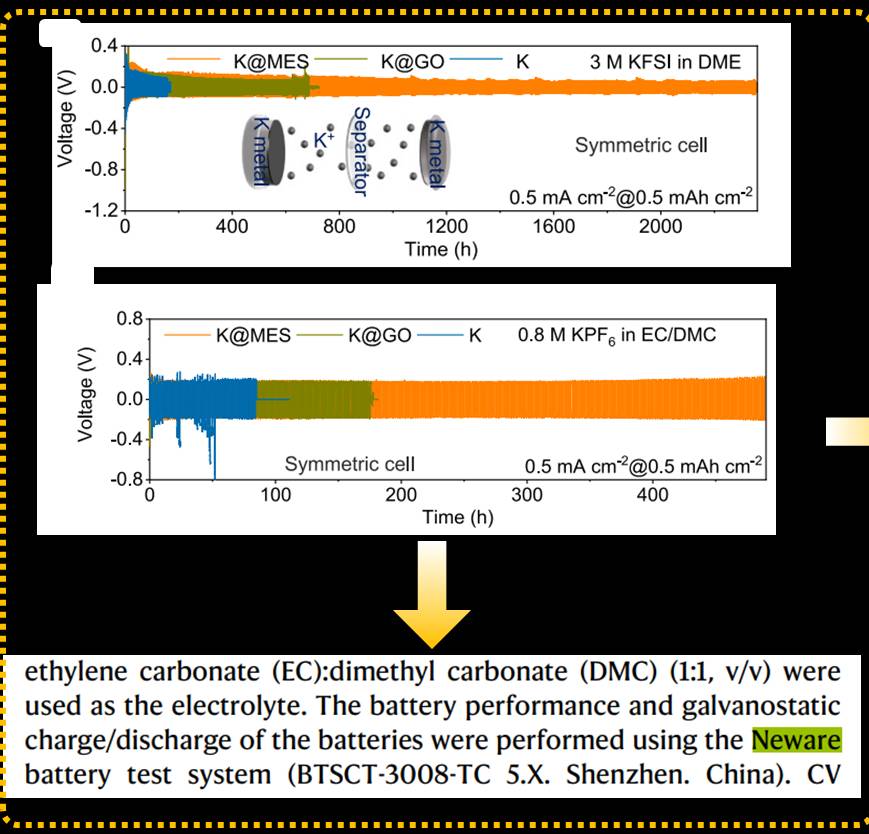
All the electrochemical performances in this study were tested using the battery testing equipment (BTSCT-3008-TC 5.X.) from NEWARE.In recent years, NEWARE has been committed to providing users with a better user experience. We have completed a comprehensive upgrade of the CT-3000 series, and the upgraded CT-4000 series offers more comprehensive features and better performance in battery testing.





The lab focuses on solid-state battery research to overcome traditional lithium batteries' safety and energy density issues, supporting environmental sustainability. It develops innovative solid-state electrolytes, refines electrode materials, and investigates ion transfer and interface stability to revolutionize battery technology.

The electric vehicle battery industry is rapidly developing, focusing on technological innovation, market competition, and sustainability. Research hotspots include solid-state batteries, new types of electrolytes, BMS optimization, and recycling technologies. The environmental adaptability, safety, and economic viability of batteries are key research areas, and the industry is expected to undergo more innovation and transformation.

We specialize in battery preparation technology research, focusing on overcoming existing energy storage challenges by innovating in electrode materials, battery chemistry, and manufacturing processes to improve performance, enhance safety, and reduce costs. Sustainability and recycling technologies for batteries are also emphasized to mitigate environmental impacts and foster the growth of green energy.