Traditional lithium-ion batteries consist of four main components: cathode, anode, electrolyte, and separator. Solid-state batteries replace the liquid electrolyte with a solid-state one. Compared to traditional lithium-ion batteries, the key difference of solid-state batteries is that the electrolyte changes from a liquid to a solid, offering both safety and high energy density. Solid-state electrolyte batteries are considered the ultimate form of lithium and sodium batteries, capable of completely solving safety issues and becoming the mainstay of new energy in the future. The working principle of solid-state batteries is similar to that of traditional liquid lithium batteries. The two ends of a traditional liquid lithium battery are the positive and negative poles, with the liquid electrolyte in the middle. The charging and discharging process is completed as lithium ions move back and forth from the cathode to the anode and then back to the cathode. The working principle of solid-state batteries is the same, where during charging, lithium ions in the cathode are detached from the crystal lattice of the active material and migrate to the anode through the solid-state electrolyte, while electrons migrate to the anode through the external circuit, where they recombine into lithium atoms, alloy, or are embedded into the anode material. The discharging process is the opposite of the charging process. By using a solid-state electrolyte instead of a liquid one, it is possible to use cathode and anode materials with higher specific capacities, while completely solving the safety issues of the battery. This is the fundamental way to achieve high energy density, safety, and long cycle life in all-solid-state lithium batteries. Therefore, solid-state batteries will be the direction of evolution for lithium-ion batteries.
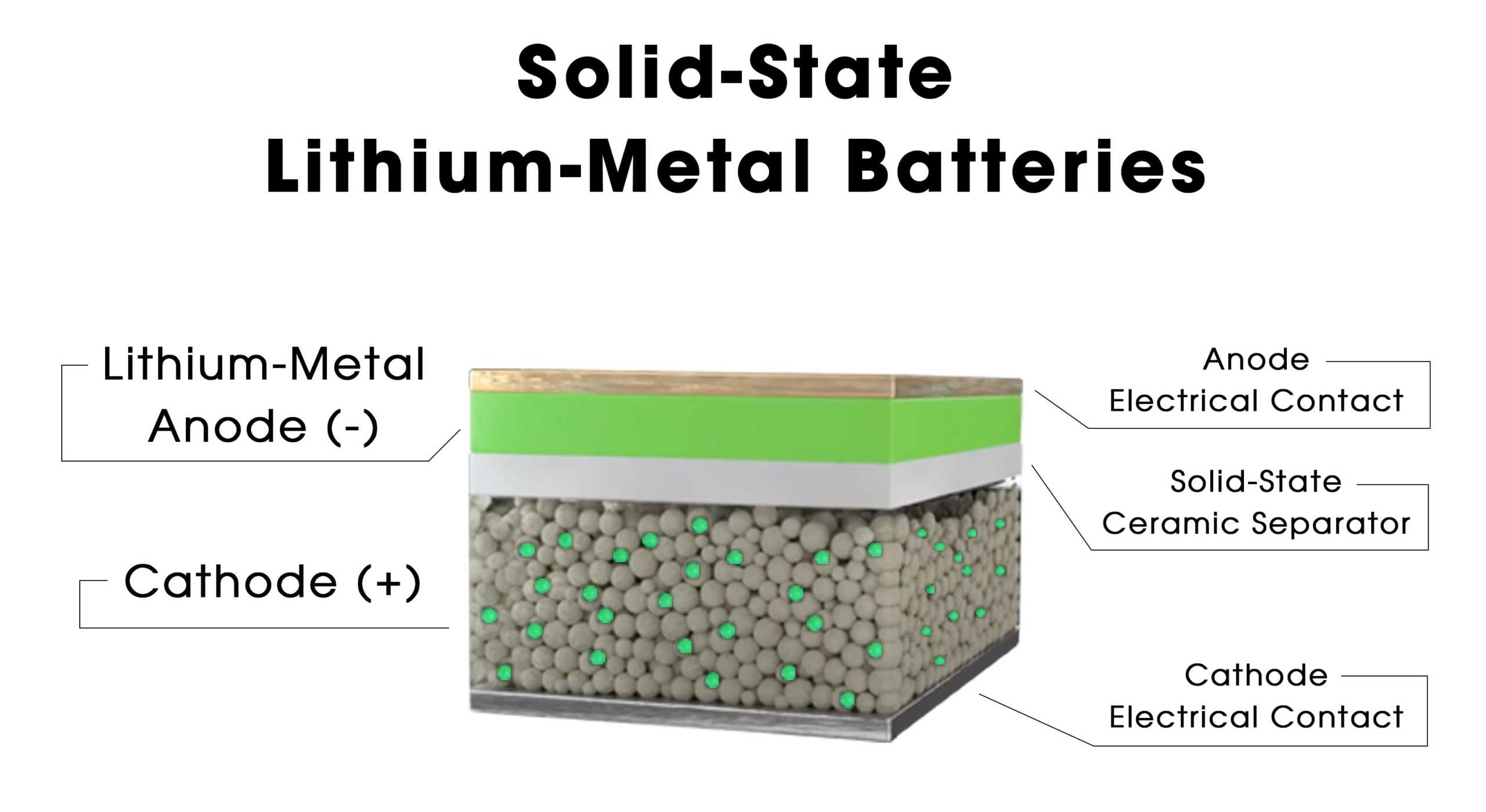
Solid-state electrolytes are the core components of solid-state lithium-ion batteries, serving as both the separator and the electrolyte of the battery. The core function of the electrolyte is to facilitate the transfer of Li+ ions between the positive and negative electrodes. An ideal solid-state electrolyte should meet characteristics such as high ionic conductivity, low interfacial impedance, structural stability, high safety, high mechanical strength, and low cost. At present, based on the type of electrolyte, they can mainly be divided into polymer solid-state electrolytes and inorganic solid-state electrolytes. The former's representative system is PEO (Poly(ethylene oxide)); the latter includes oxide, sulfide, and halide systems.
Polymer solid-state electrolytes: Flexible and lightweight, but with low potential and poor room temperature conductivity
Polymer solid-state electrolytes are systems composed of polymers with high molecular weight and lithium salts (such as LiClO4, LiAsF6, LiPF6, etc.), which are polymer electrolytes with ion transport capabilities. They exhibit ionic conductivity when coordinated with alkali metal salts. Common polymer matrices include ether-based polymers, nitrile-based polymers, siloxane-based polymers, carbonate-based polymers, and polyvinylidene fluoride-based polymers. Currently, the main material system used in the commercial field is PEO (Poly(ethylene oxide)). Under the influence of an electric field, the oxygen atoms in the PEO chain segments can continuously coordinate and dissociate with lithium ions, facilitating the migration of lithium ions. PEO also has a high solubility for lithium salts, and due to its light weight, good viscoelasticity, simple preparation process, and good interface stability with the metallic Li electrode, it is one of the earliest studied and applied systems. However, PEO tends to crystallize at room temperature, resulting in a room temperature ionic conductivity of only 10^-6 to 10^-8 S/cm (where practical applications typically require a conductivity greater than 10^-3 S/cm), necessitating operation at high temperatures of 60°C to 85°C. Moreover, the voltage threshold that PEO can tolerate is only 3.8V, which is relatively low, and it can only be paired with iron lithium cathode materials, limiting the energy density.
Oxide solid-state electrolytes: have a wide electrochemical window and good stability, with high hardness but prone to brittleness.
Oxide solid-state electrolytes, composed of oxide inorganic salts, can be divided into crystalline and amorphous electrolytes. In addition to the amorphous lithium phosphorus oxynitride (LiPON) type electrolytes used in thin-film batteries, current commercialization mainly focuses on the research of crystalline electrolyte materials. The mainstream crystalline electrolyte material systems include: garnet (LLZO) structured solid-state electrolytes, perovskite (LLTO) structured solid-state electrolytes, NASICON-type sodium superionic conductor solid-state electrolytes, and LISICON-type solid electrolytes, etc.
The general formula for garnet-type electrolytes is Li3+xA3B2O12, with the main material system being Li7La3Zr2O12, which is currently widely used; the general formula for perovskite-type electrolytes is Li3x La2/3-x TiO3, which has the advantages of structural stability, simple preparation process, and a large range of variable components, but its ionic conductivity is slightly lower; NASICON-type electrolytes can prepare high-performance Li+ solid-state electrolytes by using the NASICON framework through lithium-sodium substitution, and the mainstream materials currently are the Li1+x Alx Ti2-x(PO4)3 (lithium aluminum titanium phosphate, LATP) system. Among the aforementioned materials, LLZO has a high compatibility with lithium anodes; NASICON-type and perovskite-type electrolytes have poor electrochemical stability against metallic Li. Overall, the room temperature ionic conductivity of oxide solid-state electrolytes is relatively high, reaching 10^-5 to 10^-3 S/cm, and they have a wide electrochemical window, high chemical stability, and considerable mechanical strength, making them an ideal solid-state electrolyte material system. However, they also have the risks of high sintering temperatures and susceptibility to brittle fracture during mechanical processing.
The general chemical formula for halide electrolytes is Lia-M-Xb, which is derived from introducing high-valence transition metal cations M into lithium halides LiX (X = Br, Cl, F) to regulate the concentration of Li+ and vacancies, thereby forming compounds similar to Lia-M-Xb. Compared to oxides and sulfides, the interaction between the monovalent halide anion and Li+ is weaker and the radius is larger than that of S2− or O2−, greatly improving the room temperature ionic conductivity of the electrolyte. The theoretical ionic conductivity of the electrolyte can reach the order of 10−2 S/cm. At the same time, halides generally have a higher redox potential and are more compatible with high-voltage cathode materials, which can achieve stable cycling at high voltage windows and are considered to be very promising materials for all-solid-state lithium-ion batteries.
There are currently three common types of halide electrolytes: Lia-M-Cl6, Lia-M-Cl4, and Lia-M-Cl8 type halides, with the ionic conductivity of the first two types reaching 10-3S/cm. However, halide electrolytes are prone to phase transitions at different temperatures, which can affect conductivity, and they are easily hydrolyzed in the air, resulting in high synthesis costs. In addition, the reaction between transition metals and lithium metal leads to poor compatibility with lithium anodes.
Cathode materials for solid-state batteries mainly include: lithium cobalt oxide (LiCoO2), lithium iron phosphate (LiFePO4), lithium nickel oxide (LiNiO2), and lithium aluminum oxide (LiAlO2).
Lithium cobalt oxide (LiCoO2): A commonly used cathode material in lithium-ion batteries, it can provide high energy density and long cycle life, but there are safety concerns.
Lithium iron phosphate (LiFePO4): Compared to lithium cobalt oxide, lithium iron phosphate has better safety and longer lifespan, but lower energy density.
Lithium nickel oxide (LiNiO2): High energy density and long cycle life, but the material is expensive and has safety issues.
Lithium aluminum oxide (LiAlO2): High energy density, but the cycle life is slightly lower than that of lithium nickel oxide.
Various material combinations in solid-state electrolytes: For example, lithium manganate (LiMn2O4) and lithium titanate (Li4Ti5O12), which can provide higher safety and longer lifespan, but have relatively lower energy density.
Anode materials for solid-state batteries mainly include three types: metallic lithium, carbon materials, and silicon materials.
Metallic lithium is primarily used in solid-state lithium-ion batteries and solid-state lithium-sulfur batteries. Solid-state lithium-ion batteries are high-energy-density batteries that can be applied in fields such as electric vehicles and drones; solid-state lithium-sulfur batteries, on the other hand, are high-energy-density and high-safety batteries that can be applied in aerospace and military fields.
Carbon materials are mainly used in solid-state lithium-ion batteries. Carbon nanotubes, a common type of carbon material, have a high specific surface area and excellent electrochemical performance, making them suitable for high-performance solid-state lithium-ion batteries.
Silicon material is an emerging anode material with high specific capacity and lower cost. In solid-state batteries, silicon materials can react with solid electrolytes to form lithium ions, thereby enabling the charging and discharging of the battery. Compared to metallic lithium and carbon materials, silicon materials have a higher specific capacity, but they have poorer cycle stability and are prone to volume expansion and structural damage. Silicon materials are mainly used in solid-state lithium-ion batteries. Silicon nanowires, a common type of silicon material, have a high specific surface area and excellent electrochemical performance, making them suitable for high-performance solid-state lithium-ion batteries.
Separator materials are an important component of solid-state batteries, mainly used to isolate the positive and negative electrodes to prevent electronic conduction. The composition of separator materials mainly includes polymers, nanoscale powders, etc. Research suggests that a double-layer coating can replace the separator, with an inorganic solid-state electrolyte layer coated on both sides of the anode, and an organic polymer layer coated on the surface of the inorganic solid-state electrolyte layer. Currently, there is a view that sulfide and oxide all-solid-state batteries do not need a separator. In addition, various patents for solid-state batteries that have been made public have also proposed the concept of composite separators, such as inorganic-organic composite separators.
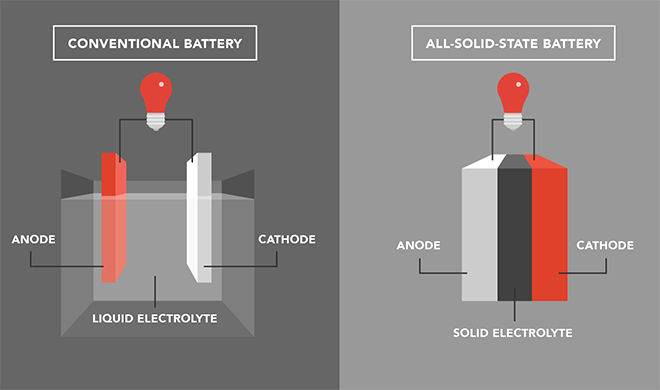
Solid-state batteries possess significant advantages of high energy density and high safety, making them the next generation of high-performance lithium batteries. In terms of performance comparison, theoretically, solid-state batteries excel in various indicators such as ionic conductivity, energy density, high-voltage resistance, high-temperature endurance, and cycle life, all of which are superior to those of liquid batteries. They combine the high energy density and high safety characteristics that traditional liquid lithium batteries cannot offer, making them the ideal batteries for electric vehicles.
Liquid lithium batteries are prone to thermal runaway. Factors such as overcharging, impact, short circuit, and immersion in water can increase the risk of thermal runaway. When the temperature rises to 90°C, the SEI film on the negative electrode surface begins to decompose, exposing the lithium-embedded carbon directly to the electrolyte, which reacts and releases heat, generating a large amount of flammable gases, and then melting the separator to form an internal short circuit. When the temperature rises to 200°C, it promotes the gasification and decomposition of the electrolyte, causing the battery to undergo intense combustion and explosion.
Compared to liquid lithium batteries, solid-state batteries have five major safety features. 1) The solid electrolyte has high mechanical strength, which can suppress the growth of lithium dendrites and prevent short circuits. 2) It is not easily flammable or explosive. 3) There are no ongoing side reactions at the interface. 4) There is no issue with electrolyte leakage or drying. 5) The lifespan at high temperatures is unaffected or better.
Energy density = operating voltage × specific capacity. The energy density of traditional liquid batteries has already approached the theoretical limit of 350Wh/kg, while solid-state batteries can achieve more than 500Wh/kg, which are not at all in the same league in terms of energy density. Solid-state batteries have a wide electrochemical window and can withstand higher voltages (above 5V), with a broader range of materials to choose from. Since the energy density of a battery is equal to the operating voltage multiplied by the specific capacity, and the overall specific capacity of the battery follows the barrel effect, it is limited by the lower end of the positive and negative electrodes.
Currently, in solid-state batteries, the specific capacity of graphite anode is 372mA•h/g, the theoretical specific capacity of silicon-based anode is 4200mA•h/g, and the theoretical specific capacity of lithium metal anode is 3860mA•h/g, all of which are significantly higher than that of the cathode. Therefore, the cathode material has become the main bottleneck for further performance improvement of lithium-ion batteries. The all-solid-state electrolyte can not only be compatible with the above high specific capacity anode materials and conventional cathode material systems but also match high specific capacity cathode materials, making the energy density reach 500Wh/kg or even higher.
Traditional liquid batteries have a relatively narrow operating temperature range. At low temperatures, the performance of liquid batteries decreases due to increased viscosity of the electrolyte, reduced ionic conductivity, increased interfacial impedance and charge transfer impedance between the electrolyte and electrodes, and reduced lithium ion migration rate. In addition, liquid batteries are limited at high temperatures due to the low flash point of the electrolyte and the low melting temperature of the separator, posing a risk of combustion. Solid-state electrolyte batteries, on the other hand, do not have the issue of electrolyte solidification at low temperatures, and are less affected and safer at high temperatures, thus having a larger operating temperature range, reaching from -40°C to 150°C, which is significantly superior to liquid batteries.
Traditional liquid batteries require the use of a separator and electrolyte, which together occupy nearly 40% of the battery's volume and 25% of its mass. Solid-state batteries replace the separator and electrolyte of liquid batteries with a solid electrolyte, allowing the distance between the anode and cathode to be reduced to just a few to several tens of micrometers, thereby significantly reducing the thickness of the battery. As a result, for the same amount of electrical charge, the volume of solid-state batteries will be smaller.
As the content of liquid electrolyte gradually decreases, the development path of solid-state batteries can generally be divided into semi-solid (5-10wt%), quasi-solid (0-5wt%), and all-solid (0wt%) stages, among which semi-solid and quasi-solid batteries use mixed solid-liquid electrolytes. Currently, on a global scale, all-solid-state batteries are mainly in the research and development and prototyping stages. The main limitations hindering the industrialization of all-solid-state batteries are: the material and manufacturing technologies are not yet mature, and the production costs are too high. The industry generally believes that it will take at least another 5 years for all-solid-state batteries to achieve large-scale industrialization. Before all-solid-state batteries officially enter the commercialization phase, semi-solid batteries may be a good transitional technical solution. Semi-solid batteries use a mixed solid-liquid electrolyte, with the content of the electrolyte in the battery ranging between 5-10%. By adding a solid electrolyte coating, its electrochemical principle is the same as that of liquid lithium batteries, and it can basically continue to use the existing mature battery manufacturing processes, which are less difficult to produce than solid-state batteries. Compared with traditional liquid lithium batteries, semi-solid batteries have significantly improved performance, with advantages including better safety, higher energy density, better flexibility, longer cycle life, wider operating temperature range, and resistance to compression and vibration. Therefore, semi-solid batteries have become a transitional technology from liquid batteries to all-solid-state batteries.
There are three main technological pathways for solid-state batteries: polymer solid-state batteries, oxide solid-state batteries, and sulfide solid-state batteries. The different technological routes of solid-state batteries are primarily distinguished by different solid electrolytes. According to the classification of solid electrolytes, there are three main technological routes: polymer electrolytes, oxide electrolytes, and sulfide electrolytes. Polymer electrolytes belong to organic electrolytes, while oxide and sulfide electrolytes belong to inorganic electrolytes.
The ideal solid electrolyte material should have high ionic conductivity, chemical and electrochemical stability towards lithium metal, be able to effectively suppress the formation of lithium dendrites, have low manufacturing costs, and not require the use of rare metals. However, the three main technological routes each have their pros and cons, and there is no single one that can meet all the above requirements at the same time, and there are still certain difficulties in technological breakthroughs. Overall, sulfide electrolytes are considered to have the most potential for development in all-solid-state batteries.
Polymer electrolytes: The advantages of polymers are their ease of processing, compatibility with existing electrolyte production equipment and processes, and good mechanical properties. Their disadvantages include: (1) low ionic conductivity, requiring heating to 60°C to enable normal charging and discharging; (2) poor chemical stability, not suitable for high-voltage cathode materials, and prone to combustion at high temperatures; (3) a narrow electrochemical window, and when the potential difference is too large (>4V), the electrolyte is prone to electrolysis, limiting the performance of polymers.
Oxide electrolytes: Their advantages lie in better conductivity and stability, with higher ionic conductivity than polymers, thermal stability up to 1000°C, and good mechanical and electrochemical stability. Their disadvantages include: (1) compared to sulfides, their ionic conductivity is relatively low, which can lead to a series of issues such as limited capacity and rate performance in the performance improvement process of oxide solid-state batteries; (2) oxides are very hard, leading to rigid interface contact issues in solid-state batteries, and under simple room temperature cold pressing, the porosity of the battery is very high, which may prevent the battery from functioning properly.
Sulfide electrolytes: They have the highest ionic conductivity, good mechanical properties, and a wide electrochemical stability window (above 5V), showing excellent performance, and have the greatest potential for development in all-solid-state batteries. Their disadvantages include: (1) unstable interfaces, prone to side reactions with cathode and anode materials, resulting in high interface impedance and increased internal resistance; (2) in terms of manufacturing processes, the preparation of sulfide solid-state batteries is more complex, and sulfides are prone to react with water and oxygen in the air to produce highly toxic hydrogen sulfide gas.
Among them, polymer electrolytes have developed most rapidly, with relatively mature technology, and are the earliest to promote commercial applications, having achieved small-scale mass production. However, they have the disadvantage of low conductivity and a lower performance limit, and have not yet been widely adopted. Oxide electrolytes show a more balanced performance in all aspects and are currently progressing rapidly. Sulfide electrolytes have higher conductivity and the most excellent performance, making them most suitable for electric vehicles, with great commercial potential, but the research difficulty is also high, and how to maintain high stability remains to be solved. Achieving technological breakthroughs in key issues of solid electrolytes will likely accelerate the process of industrialization.
The development of solid-state electrolytes faces three major scientific issues. The mechanisms of ion transport in solid-state electrolytes, the growth mechanism of lithium dendrites in lithium metal anodes, and the failure mechanisms of multi-field coupling systems are the core scientific issues faced by the development of solid-state batteries. Solving these problems is essential for creating new solid-state electrolyte materials, optimizing the physicochemical performance of solid-state batteries, and promoting the development of solid-state batteries.
The comprehensive performance of solid-state battery electrolytes is difficult to balance. In terms of material characteristics, whether it is polymers, oxides, or sulfides, their overall performance as solid-state electrolytes is not satisfactory. For example, polymer electrolytes are easy to process and have low production difficulty, but they have low ionic conductivity, which affects charging and discharging performance. Oxide and sulfide electrolytes have higher conductivity, safety, and mechanical strength, but their manufacturing difficulty is greater and costs are higher.
Solution approach: Composite electrolytes integrate the advantages of multiple materials. Therefore, the idea of composite materials is to use different materials in combination, hoping to take advantage of both. Polymer/polymer composite electrolyte materials have stronger processability, and both mechanical strength and ionic conductivity are improved. For polymer/inorganic (oxide/sulfide) composite electrolyte materials, they combine the characteristics of polymers and oxides/sulfides, achieving a comprehensive integration of high strength and better flexibility, conductivity, and easy preparation.
The bottleneck of all-solid-state batteries mainly lies in the slower charging and discharging speed and the faster capacity decay. Ionic conductivity is the key to improving the charging and discharging speed of all-solid-state batteries. The ion transport performance in solid-state electrolytes is jointly determined by the transport process of ions in the bulk and at the interface. Compared with liquid electrolytes, the interaction force between ions in solid-state electrolytes is strong, and the ion migration energy barrier is more than ten times that of liquids, resulting in low ionic conductivity.
High mechanical strength solid-state electrolytes still find it difficult to completely suppress the growth of lithium dendrites and achieve uniform deposition of lithium metal. Studies have shown that inorganic solid-state electrolytes with high shear modulus cannot completely prevent lithium dendrites from penetrating through the solid-state electrolyte. Lithium dendrites are still an important factor hindering the practical application of all-solid-state batteries. For example, the shear modulus of oxide solid-state electrolytes is more than ten times that of lithium metal (above 50GPa), and the growth of lithium dendrites can still cause short circuits in solid-state batteries.
The reduction in stability caused by solid-solid interface contact is the main reason for battery failure. The interface of solid-state batteries is solid-solid contact, and the conductivity is often hindered by high contact resistance at the interface between the electrode and the electrolyte. High impedance increases overpotential, leading to capacity decay and reduced energy density. The main sources of high impedance at the interface are: (1) the interface issue between the solid-state electrolyte and the anode; (2) the interface issue between the solid-state electrolyte and the composite cathode; (3) the micro-interface issue between the cathode active material and the solid-state electrolyte within the composite cathode.
Solution approach: Interface engineering and modification, achieving improvement through two dimensions of materials and processes. Material dimension: Select Li metal anode and coated composite cathode. On the anode side, using Li alloys with smaller volume changes as anodes to alleviate anode expansion issues, macroscopic interface problems, and selecting more stable solid-state electrolytes to reduce the occurrence of side reactions at the interface. At the micro-interface of the composite cathode, surface coating can be used to reduce interface stress and improve the efficiency of ion and electron transport.
Process dimension: Macroscopic interface issues, by increasing the pressure during the manufacturing process to eliminate pores and enhance interface contact, or by in-situ solidification, injecting liquid into the solid-state battery, and after sealing, solidifying the liquid by heating or other means to enhance the interface contact between the solid-state electrolyte and the electrode.
The supply chain for raw materials and the manufacturing equipment for solid-state batteries are not yet perfected. Currently, some raw materials for solid-state batteries have not been mass-produced, and the overall industry chain is not yet complete, resulting in high battery manufacturing costs. Moreover, as a new type of battery, the manufacturing process for solid-state batteries lacks specific equipment, such as sintering, vacuum, drying rooms, and specific atmospheres, all of which will increase the manufacturing costs of solid-state batteries. The cost of electrode materials for solid-state batteries is high. Oxide cathode materials are mainly made from inorganic materials such as aluminum oxide and titanium oxide; sulfide cathode materials are composed of sulfur, sulfides, and polymers; and polymer cathodes are made up of various polymer compounds such as polycarbonates and cellulose. For example, the high cost of germanium hinders mass production for the LGPS-type sulfide electrolyte, which has considerable performance.
Solution approach: Semi-solid first, scale to reduce material costs. Semi-solid-state batteries are relatively mature in technology and are closer to liquid lithium-ion batteries. If the industrialization of semi-solid-state batteries can be achieved, then with the increase in production capacity of the corresponding solid-state electrolytes and the reduction of raw material costs, as well as process optimization, the cost of raw materials and production is expected to decrease.
Toyota + Panasonic (Japan)
Main technology route: Sulfide
Progress: Since 2004, Toyota has been developing all-solid-state batteries, accumulating rich technology and patents; in January 2019, it announced the establishment of a new company with Panasonic to develop solid-state batteries by 2020, and exhibited a sample of solid-state batteries in May; in 2020, it launched a new energy vehicle equipped with solid-state batteries, planning for mass production by 2025.
Hitachi Shipbuilding (Japan)
Main technology route: Sulfide
Progress: Launched all-solid-state battery (AS-LiB), first applied in the aerospace field, planning to apply to the automotive market after 2025.
Samsung SDI + SKI + LG Chem (South Korea)
Main technology route: Sulfide
Progress: In 2017, Samsung SDI exhibited a solid-state battery; in 2018, the three companies cooperated and established a 10 billion won fund to jointly invest in solid-state lithium batteries and other new generation battery technologies, accelerating the commercialization process of core technologies; in 2020, Samsung SDI released the latest scientific research results of solid-state batteries, the silver-carbon-based all-solid-state battery can achieve a high energy density of 900Wh/L, a long cycle life of over 1000 cycles, and a Coulomb efficiency of 99.8%, allowing the car to drive 800 kilometers after one charge.
Bollore (France)
Main technology route: Polymer
Progress: First to use electric vehicles equipped with solid-state batteries, launched the Bluecar in 2011, equipped with a 30kWh polymer (LMP) battery.
Solid Power (USA)
Main technology route: Polymer
Progress: Derived from the scientific research achievements of the University of Colorado at Boulder, has been invested by BMW, Hyundai, Samsung, and other companies, in 2019, it cooperated with Ford to develop a new generation of electric vehicle solid-state batteries; in October 2020, Solid Power announced the production and delivery of its first-generation 2Ah all-solid-state battery (ASSB), with an energy density of 320Wh/kg, the product is ready to be launched in the market in 2021, and is planned to be applied in the automotive field in 2026.
Solid energy system (USA)
Main Technology Route: Polymer
Progress: Founded by researchers from MIT, raised $30 million from companies such as General Motors; in 2020, SES cooperated with Foxconn and CATL in the power battery field, and planned to launch solid-state batteries in 2024; in 2021, it reached a cooperative relationship with General Motors, as part of the agreement, the two companies plan to build a prototype factory in Woburn, Massachusetts, with the goal of having a high-capacity pre-production battery before 2023.
Ionic Materials (USA)
Main technology route: Polymer
Progress: In 2018, it received investment from the Renault-Nissan-Mitsubishi alliance; by 2025, Renault's electric vehicles plan to use solid-state batteries with zero cobalt content, supported by Ionic Materials.
Quantum Scape (USA)
Main technological approach: Oxide
Progress: Received funding from Volkswagen, with Volkswagen holding a 5% stake in 2014; additional investment of $100 million in June 2018; further investment of $200 million in June 2020; the collaborative goal is to achieve mass production of solid-state batteries before 2025.




The lab focuses on solid-state battery research to overcome traditional lithium batteries' safety and energy density issues, supporting environmental sustainability. It develops innovative solid-state electrolytes, refines electrode materials, and investigates ion transfer and interface stability to revolutionize battery technology.

The electric vehicle battery industry is rapidly developing, focusing on technological innovation, market competition, and sustainability. Research hotspots include solid-state batteries, new types of electrolytes, BMS optimization, and recycling technologies. The environmental adaptability, safety, and economic viability of batteries are key research areas, and the industry is expected to undergo more innovation and transformation.

Specializing in battery preparation technology research, the focus is on overcoming existing energy storage challenges by innovating in electrode materials, battery chemistry, and manufacturing processes to improve performance, safety, and reduce costs. Sustainability and recycling technologies for batteries are also emphasized to mitigate environmental impacts and foster the growth of green energy.