Since the 1990s, lithium-ion batteries have developed into the most mature and widely used battery technology route. With the increasing requirements of the market for battery energy density, safety, and economy, 'solid-state batteries' that use solid electrodes and solid electrolytes and have higher energy density and safety have emerged.
Traditional lithium-ion batteries include four major components: positive electrode, negative electrode, electrolyte and separator. Solid-state batteries replace the electrolyte with solid electrolyte. The key difference between solid-state batteries and traditional lithium-ion batteries is that the electrolyte changes from liquid to solid, taking into account safety, high energy density and other properties. Solid electrolyte battery is the final form of lithium sodium battery, which can completely solve the safety problem and is the protagonist of the second half of the new energy.
High safety: All-solid-state lithium batteries use solid electrolytes, which are generally synthesized by organic and inorganic compounds. The melting point and boiling point are high, and most of the materials are non-flammable. At the same time, the solid electrolyte film is dense and non-porous, and the mechanical strength is high, which effectively suppresses the short-circuit problem caused by the negative lithium dendrite puncture.
High energy density: The specific capacity of lithium metal is high, which is close to 10 times that of graphite anode. Even if lithium metal only exerts 50% effective capacity, it is much higher than graphite and silicon-based anodes.
The application temperature range is wide: The decomposition temperature of solid electrolyte is generally high. The polymer organic solid electrolyte is generally used at a temperature of more than 150 degrees Celsius, and the maximum temperature of inorganic solid electrolyte is expected to increase to 300 degrees Celsius, which broadens the application of lithium battery in the field of high temperature.
Design diversification: Due to the fluidity of the electrolyte, the external morphology and internal structure design of the battery will be limited. The solid electrolyte reduces the steps of liquid injection, the preparation process is simplified, and the battery design is diversified.
Generally speaking, there are mainly positive electrode, negative electrode, separator and electrolyte inside the battery, while the electrolyte in the traditional liquid battery system accounts for about 25 wt%.
Solid-state batteries are generally classified into three types: semi-solid, quasi-solid, and all-solid batteries. They can be collectively referred to as solid-state batteries, and the difference is that their liquid contents are 5-10 wt%, 0-5 wt%, and 0 wt%, respectively. Whether the separator exists depends on the liquid content in the system, and the electrolyte has become the main factor restricting the development of solid-state batteries.
As a key factor in solid-state lithium batteries, the performance of solid-state electrolytes is crucial. So far, solid-state electrolytes are mainly divided into four categories, namely oxide solid-state electrolytes, sulfide solid-state electrolytes, halide solid-state electrolytes and polymer solid-state electrolytes.
·Oxide solid electrolyte: LiPON (LixPOyNz), LLZO (Li7La3Zr2O12), LATP (Li1.3Al0.3Ti1.7(PO4)3), LLTO (Li0.33La0.56TiO3) are the main components. The advantages of oxide solid electrolyte are: high ionic conductivity at room temperature, reaching 10-5~10-3 S/cm, wide electrochemical window, high chemical stability and high mechanical strength. However, there are also risks of high sintering temperature and brittle fracture during machining. Figure 1 is the crystal structure diagram of LLZO.
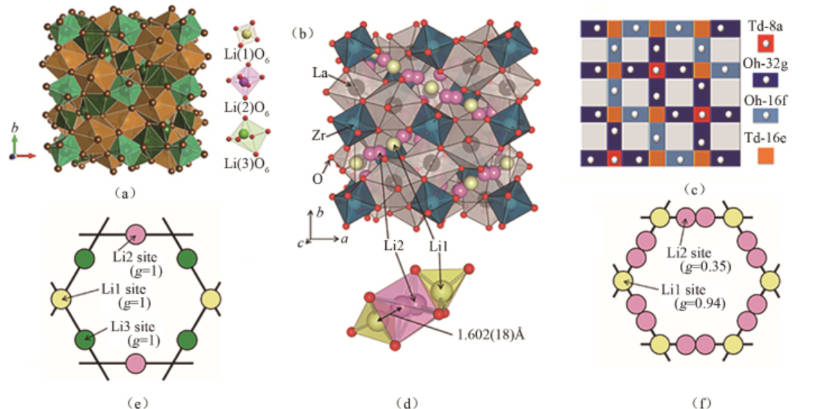
Figure-1-The-crystal-structure-diagram-of-LLZO
·Sulfide solid electrolyte: It is derived from the oxide solid electrolyte, that is, the oxygen element in the oxide body of the electrolyte is replaced by the sulfur element. Compared with O2-, the radius of S2- is larger, resulting in a larger ion conduction channel; the smaller electronegativity and the smaller interaction with Li+ greatly improve the ionic conductivity of the electrolyte at room temperature.
The sulfide solid electrolyte is represented by Li2SiP2S12, Li4-xAl-yByS4 (A=Ge, Si, B=P, Al, Zn, etc.) and Li6PS5X (X=Cl, Br, I). The main advantages are: compared with the oxide solid electrolyte, the electrolyte has a wide range of composition changes, higher ionic conductivity, and ionic conductivity can reach 10-4~10-2 S/cm. However, the electrolyte also has the following disadvantages:
(1) Sulfide will rapidly hydrolyze in the presence of air to produce H2S gas, so the electrolyte synthesis needs to be carried out in an inert atmosphere, resulting in high cost of research and development, manufacturing, transportation and storage.
(2) Because S2- is easier to be oxidized than O2-, sulfide electrolyte is easier to be oxidized and decomposed at high voltage, and the electrochemical window is narrower. Figure 2 is the structure diagram of Li6PS5Cl material.
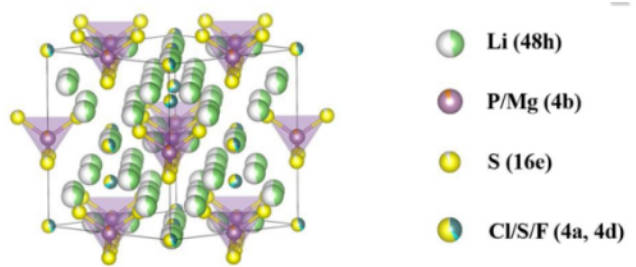
Figure-2-The-structure-diagram-of-Li6PS5Cl
·Halide solid electrolyte: The chemical formula is LiaMXb, which is derived from the introduction of high valence transition metal element M cations into lithium halide LiX (X=Br, Cl, F) to adjust Li+ and vacancy concentration to form LiaMXb-like compounds.
In general, halide electrolytes also have high ionic conductivity at room temperature. The theoretical ionic conductivity of the electrolyte can reach the order of 10-2 S/cm. There are three types of common halide electrolytes: LiaMCl4, LiaMCl6 and LiaMCl8. The ionic conductivity of the first two types can reach 10-3 S/cm. However, halide electrolytes are prone to phase transition at different temperatures, which affects the conductivity, and are easily hydrolyzed in air, so the synthesis cost is high. In addition, the reaction between transition metal and lithium metal leads to poor compatibility of lithium anode. Figure 3 is the evolution of the basic LiCl structure to other structures in the halide solid electrolyte.
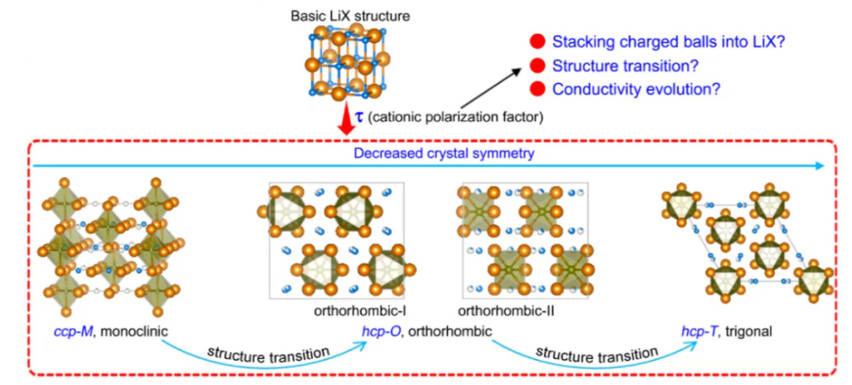
Figure-3-The-evolution-of-the-basic-LiCl-structure-to-other-structures-in-halide-solid-electrolytes
·Polymer solid electrolyte: It is a system composed of high molecular weight polymers and lithium salts (such as LiCIO4, LiAsF6, LiPF6, etc.), a polymer electrolyte with ion transport capacity, and alkali metal salt coordination with ionic conductivity.
The general polymer matrices include ether-based polymers, nitrile-based polymers, siloxane-based polymers, carbonate-based polymers, and vinylidene fluoride polymers. At present, the main material system suitable for the commercial field is PEO (polyethylene oxide). Under the action of electric field, the oxygen atoms and lithium ions in the PEO segment can be continuously coordinated and dissociated to achieve the migration of lithium ions. At the same time, PEO has a high solubility of lithium salts, and because of its light weight, good viscoelasticity, simple preparation process, not easy to crack, and good interface stability with metal Li electrodes, it is one of the earliest research and earliest application systems.
As a key factor in solid-state lithium batteries, solid-state electrolytes have developed rapidly since 1957. Figure 4 shows its development process.
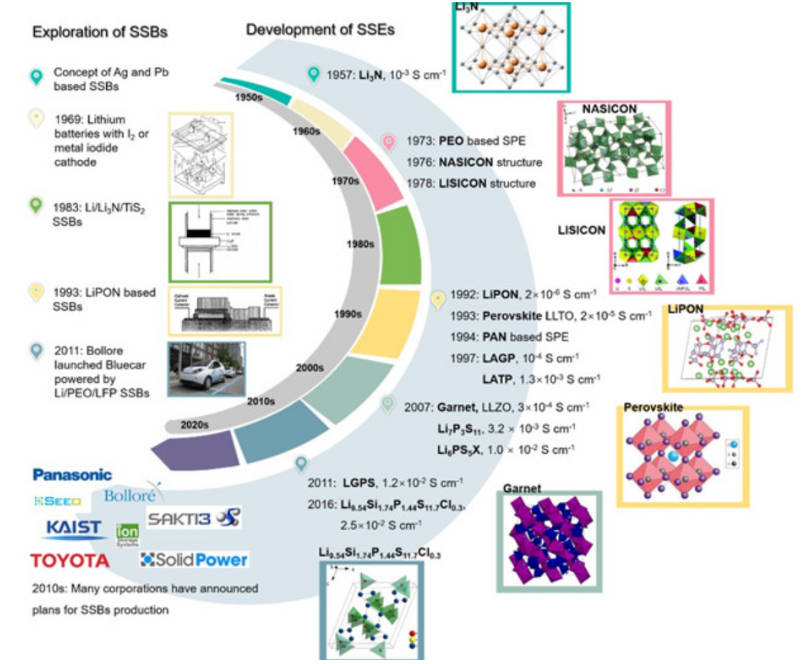
Figure-4-The-development-history-of-solid-state-lithium-battery
However, although the development of solid-state lithium batteries is relatively rapid, it still faces many difficulties and challenges:
The core technical difficulty of solid-state lithium batteries lies in electrolytes: As previously described, electrolytes are an important medium for lithium ion transport and are critical to battery performance. The defects of the lithium metal anode, the failure of the solid electrolyte interface in contact with the lithium metal, the poor mechanical stability of the active cathode material and the solid composite cathode material, and the solid electrolyte materials based on different technical systems have their own defects or shortcomings. The stability of the electrolyte interface is crucial to the long cycle life of all-solid-state lithium batteries.
The electrolyte from liquid to solid is full of challenges: in the selection of solid electrolyte and cell design, the problem of solid phase interface contact and volume expansion during the cycle needs to be continuously solved. Among them, the flexibility of oxide materials is relatively poor, and poor interface contact will lead to the increase of interface impedance ; the polymer has the problem of low conductivity, which is 4~5 orders of magnitude lower than the current liquid electrolyte. Sulfide solid-state batteries are faced with problems such as electrolyte sensitivity to air, harsh manufacturing conditions, expensive raw materials, and immature large-scale production technology.
The batch manufacturing of solid-state lithium batteries faces engineering and cost problems: can the development process from laboratory to factory be accelerated? Can a standard solid-state battery be produced on the production line? Does the performance meet people 's expectations? Does it have commercial applications in the automotive field? These are extremely critical standards. Despite the outstanding advantages of high energy density and high safety, as far as the current research and development progress of the industry is concerned, solid-state batteries at this stage have more or less shortcomings that cannot be broken through in a short time in terms of material cost, processing cost, and mass production capacity.



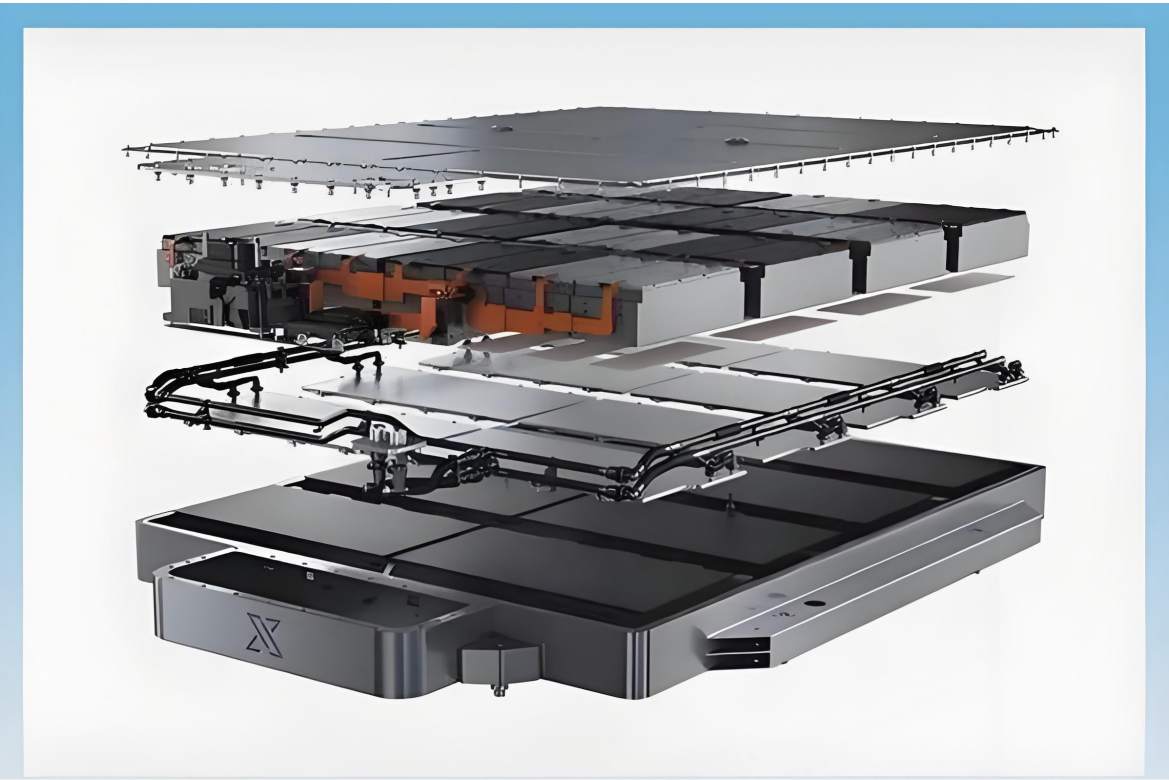

Technology
December 04, 2025

Technology
November 30, 2025

The lab focuses on solid-state battery research to overcome traditional lithium batteries' safety and energy density issues, supporting environmental sustainability. It develops innovative solid-state electrolytes, refines electrode materials, and investigates ion transfer and interface stability to revolutionize battery technology.

The electric vehicle battery industry is rapidly developing, focusing on technological innovation, market competition, and sustainability. Research hotspots include solid-state batteries, new types of electrolytes, BMS optimization, and recycling technologies. The environmental adaptability, safety, and economic viability of batteries are key research areas, and the industry is expected to undergo more innovation and transformation.

We specialize in battery preparation technology research, focusing on overcoming existing energy storage challenges by innovating in electrode materials, battery chemistry, and manufacturing processes to improve performance, enhance safety, and reduce costs. Sustainability and recycling technologies for batteries are also emphasized to mitigate environmental impacts and foster the growth of green energy.