
Deep cycle batteries are specialized rechargeable batteries crafted for sustained power delivery and frequent cycling through deep discharges of 80-100% without significant degradation. Unlike starter batteries, which provide intense, short-lived bursts for engine ignition, deep cycle batteries are designed for applications requiring consistent power over time. They are ideal for use in renewable energy systems, marine crafts, RVs, and off-grid setups, where their ability to endure regular, substantial discharges and subsequent recharges is particularly beneficial.
Deep cycle batteries come in several types, including Sealed Lead Acid (SLA), Lithium-ion, Flooded Lead-Acid (FLA), Absorbent Glass Mat (AGM), Gel, Nickel-Iron (NiFe), and Lithium Iron Phosphate (LiFePO4). Each type offers distinct advantages and is suited to various applications such as renewable energy systems, marine use, and off-grid power solutions.
Sealed Lead Acid (SLA) deep cycle battery, also known as a valve-regulated lead-acid (VRLA) battery, consists of lead dioxide (PbO₂) as the positive plate and sponge lead (Pb) as the negative plate, both immersed in an electrolyte solution of sulfuric acid (H₂SO₄). Unlike traditional flooded lead-acid batteries, SLAs are sealed and maintain their electrolyte in either a gel form (Gel batteries) or absorbed in a fiberglass mat (Absorbent Glass Mat, or AGM batteries). The working principle involves the electrochemical reaction between the lead plates and the sulfuric acid, where during discharge, lead dioxide reacts with sulfuric acid to form lead sulfate and water, releasing electrons that provide electrical power. During charging, the process is reversed, converting lead sulfate back into lead dioxide and sulfuric acid, restoring the battery to its charged state. SLAs are designed to be maintenance-free, as the sealed nature prevents the loss of electrolyte and minimizes the emission of gases, thanks to the valve regulation that allows gas release only when internal pressure exceeds a certain limit.
One of the primary advantages of SLA batteries is their maintenance-free operation, which eliminates the need for regular electrolyte checks and top-ups, making them highly convenient for users. Additionally, the sealed design enhances safety by reducing the risk of acid spills and minimizing the release of hydrogen and oxygen gases, thereby mitigating the risk of explosions or corrosion. SLAs also tend to have a lower self-discharge rate compared to flooded batteries, allowing them to retain their charge for longer periods when not in use. However, there are some disadvantages to consider. SLAs generally have a higher initial cost than flooded lead-acid batteries, which can be a factor for cost-sensitive applications. They are also more sensitive to overcharging and deep discharging, which can significantly reduce their lifespan if not managed properly. Furthermore, while SLAs are less prone to leakage, they are heavier and less energy-dense than some modern battery technologies like lithium-ion, which can be a limitation in applications where weight and space are critical considerations. Overall, SLA deep cycle batteries are a robust and reliable choice for many applications, offering a good balance of performance, safety, and convenience, albeit with some trade-offs in cost and energy density.
lithium-ion deep cycle battery is composed of several key components: the anode (typically made of graphite), the cathode (commonly made from lithium cobalt oxide, lithium iron phosphate, or other lithium metal oxides), the electrolyte (a lithium salt dissolved in an organic solvent), and a separator that prevents direct contact between the anode and cathode while allowing ions to pass through. The working principle of a lithium-ion battery involves the movement of lithium ions between the anode and cathode during charge and discharge cycles. During charging, lithium ions move from the cathode to the anode through the electrolyte, and electrons flow through an external circuit to balance the charge, effectively storing energy. When discharging, the process reverses: lithium ions move back to the cathode, and electrons flow through the external circuit to provide power to the connected load.
Lithium-ion deep cycle batteries offer several advantages. They have a high energy density, meaning they can store more energy in a smaller and lighter package compared to traditional lead-acid batteries. This makes them highly suitable for applications where weight and space are critical factors, such as in electric vehicles, portable electronics, and renewable energy storage systems. They also have a long cycle life, often lasting several thousand charge-discharge cycles, which translates to a longer overall lifespan. Additionally, lithium-ion batteries exhibit high charge and discharge efficiency, typically around 95%, meaning less energy is lost during the charging process. They also have a low self-discharge rate, allowing them to retain their charge for extended periods when not in use.
However, there are some disadvantages to consider. Lithium-ion batteries are generally more expensive upfront compared to other battery technologies like lead-acid, which can be a significant factor for large-scale applications. They require sophisticated battery management systems (BMS) to monitor and manage parameters like voltage, temperature, and state of charge to ensure safe and optimal performance, which adds to the complexity and cost. Additionally, lithium-ion batteries can pose safety risks if damaged or improperly handled, as they can potentially catch fire or explode due to thermal runaway. Despite these drawbacks, the superior performance characteristics of lithium-ion deep cycle batteries make them a preferred choice for many modern applications, offering a compelling combination of efficiency, longevity, and energy density.
Flooded lead-acid (FLA) deep cycle batteries consist of positive plates made of lead dioxide (PbO₂) and negative plates made of sponge lead (Pb), submerged in an electrolyte solution of sulfuric acid (H₂SO₄) diluted with water. The plates are typically separated by microporous separators to prevent short circuits while allowing ionic movement. The working principle of FLA batteries involves electrochemical reactions where, during discharge, lead dioxide on the positive plate reacts with sulfuric acid to form lead sulfate (PbSO₄) and water (H₂O), while the sponge lead on the negative plate also forms lead sulfate. This reaction releases electrons, which flow through an external circuit to provide electrical power. During charging, an external power source reverses the reaction, converting lead sulfate back into lead dioxide and sponge lead, and regenerating the sulfuric acid in the electrolyte.
The primary advantages of FLA deep cycle batteries include their cost-effectiveness and robustness. They are generally less expensive upfront compared to other types of deep cycle batteries, making them a popular choice for large-scale applications where initial cost is a significant factor. Additionally, FLA batteries can handle a large number of charge and discharge cycles if maintained properly, offering a reliable and durable power source. However, there are several disadvantages associated with FLA batteries. They require regular maintenance, including checking and topping up the electrolyte levels and ensuring proper ventilation to expel gases produced during charging, such as hydrogen and oxygen. These gases can be explosive, necessitating careful handling and storage. FLA batteries are also heavier and bulkier compared to modern alternatives like lithium-ion batteries, which can be a limitation in applications where space and weight are critical considerations. Furthermore, the efficiency of FLA batteries is lower, with higher self-discharge rates and less efficient energy conversion, leading to more frequent maintenance and potential energy losses. Despite these drawbacks, FLA deep cycle batteries remain a widely used and reliable technology, particularly in applications where cost and durability are prioritized over maintenance convenience and energy efficiency.
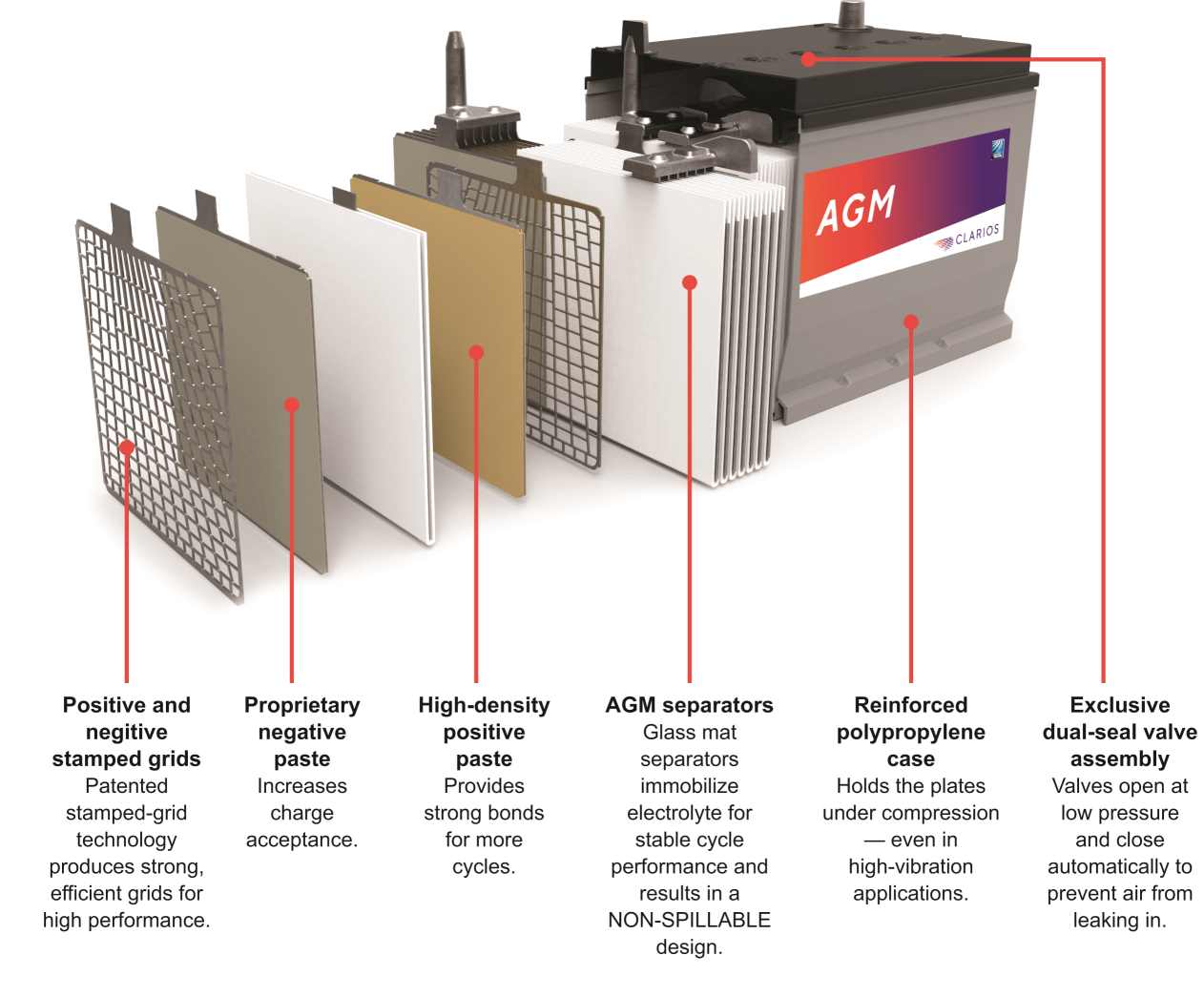
Absorbent Glass Mat (AGM) deep cycle batteries are a sophisticated class of lead-acid batteries that have gained significant popularity due to their performance and safety features. The composition of an AGM battery involves lead plates, each filled with paste made from lead oxide and a small amount of antimony or calcium, which serve as the electrodes. These plates are separated by a unique component, the absorbent glass mat, which is a thin, porous fiberglass material that soaks up the electrolyte solution, preventing it from spilling while allowing for ion flow.
The working principle of AGM batteries centers around the electrochemical reactions that occur within the lead plates and the electrolyte. During discharge, chemical energy is converted into electrical energy as the lead in the plates reacts with the sulfate from the electrolyte to form lead sulfate. The process is reversed during charging, restoring the lead plates and replenishing the electrolyte.
AGM batteries offer several advantages. They have a low internal resistance, enabling fast charging rates, and can withstand a high Depth of Discharge (DoD) of up to 80%. Their design allows for excellent recovery performance and low-temperature startup capabilities. Moreover, they are leak-proof, low-maintenance, and vibration and shock-resistant, which is invaluable in applications like motor vehicles and marine equipment.
However, there are also disadvantages to consider. AGM batteries tend to be more expensive than flooded batteries, and they have a lower tolerance to overcharging. They are also heavier compared to other sealed batteries like gel batteries, which can be a disadvantage in certain applications where weight is a critical factor.
In terms of application scenarios, AGM deep cycle batteries are incredibly versatile. They are commonly used in various settings where both high power and deep cycling capabilities are required. Examples include starter batteries for vehicles, providing the necessary power to start engines, as well as in renewable energy systems, such as solar and wind power setups, where they store and discharge energy over long periods. They are also popular in marine environments due to their robustness and resistance to corrosion, and in off-grid power systems where a reliable and steady power supply is essential.
Nickel-iron (NiFe) deep cycle batteries are a type of rechargeable battery that dates back to the early 20th century. They consist of an anode made of iron, a cathode made of nickel oxide-hydroxide, and an electrolyte composed of potassium hydroxide. The NiFe battery operates on the principle of electrochemical redox reactions; during discharge, iron at the anode oxidizes, while nickel oxide at the cathode reduces. The reverse occurs during charging, restoring the battery to its original state.
These batteries are renowned for their exceptional durability and resistance to overcharging and deep discharging, as well as their ability to withstand high temperatures and vibrations . The iron anode is particularly resistant to corrosion, contributing to the battery's long service life, which can span thousands of charge cycles and multiple decades .
However, NiFe batteries also have some drawbacks. They suffer from a relatively low energy density, typically around 50Wh/kg, and are more expensive compared to lead-acid batteries. Additionally, they exhibit a high self-discharge rate, which can reach up to 40% per month if left unused . Low-temperature performance is another concern, as the battery's efficiency can decrease significantly in colder conditions .
Despite these limitations, NiFe batteries have specific applications where their robustness and longevity are highly valued. They are used in railroad signaling systems, material handling equipment like forklifts, and in mining operations, where their ability to endure harsh conditions and resist overcharging is critical . Furthermore, due to their environmental friendliness and low maintenance requirements, there is a resurgence of interest in NiFe batteries for large-scale energy storage solutions, especially in scenarios where sustainability and long-term reliability are prioritized over energy density and initial cost .
Lithium iron phosphate (LiFePO4) deep cycle batteries are a type of lithium-ion battery that have gained significant attention for their high safety, longevity, and environmental friendliness. These batteries consist of a lithium iron phosphate cathode, a graphite anode, and an electrolyte that is typically a lithium salt in an organic solvent. The LiFePO4 battery operates using lithium ions that move between the anode and cathode through the electrolyte during charge and discharge cycles. The cathode, made of the LiFePO4 compound, provides a stable platform for lithium ions to be inserted and extracted, which contributes to the battery's excellent cycle life and thermal stability.
One of the key advantages of LiFePO4 batteries is their high energy density, which allows for a greater amount of energy to be stored in a smaller and lighter package compared to traditional lead-acid batteries. They also have a flat discharge curve, ensuring a consistent voltage output until the battery is nearly depleted. These batteries are known for their rapid charge capability and low self-discharge rate, typically losing only about 5-10% of their charge per month when idle.
However, LiFePO4 batteries also have some disadvantages. They generally have a lower nominal voltage compared to other lithium-ion batteries, which can affect their compatibility with certain applications. Additionally, while they are safe, they can still be susceptible to damage under extreme conditions, such as overcharging or crushing.
LiFePO4 batteries are widely used in various applications due to their robust performance and safety profile. They are commonly found in electric vehicles and solar energy storage systems, where their ability to handle deep cycles and resist degradation over time is particularly beneficial. In the electric vehicle market, LiFePO4 batteries provide a reliable power source for both new vehicles and conversions of existing models. For renewable energy systems, they offer a stable storage solution that can withstand the daily charge and discharge cycles, ensuring a consistent energy supply. Furthermore, their low self-discharge characteristic makes them ideal for backup power applications, where they can remain ready for use over extended periods without significant loss of charge.
Deep cycle batteries and standard batteries, often categorized as starter batteries, differ significantly in design, chemistry, and application. Deep cycle batteries are engineered to withstand frequent and substantial discharges, typically down to 80% of their capacity, followed by recharges. They feature thicker plates and are constructed to endure the physical demands of this cycle, ensuring a longer service life with hundreds to thousands of cycles. Common chemistries for deep cycle batteries include lead-acid in flooded, absorbed glass mat (AGM), or gel formats, as well as advanced lithium-ion variants. These batteries are ideal for continuous power supply needs such as in renewable energy systems, electric vehicles, marine crafts, and recreational vehicles, where a steady output is required over an extended period.
In contrast, normal batteries, or starter batteries, are specifically designed to deliver a high-current burst for a short duration, such as starting an engine. They usually have thinner plates and are optimized for high-intensity, short-term power delivery rather than deep cycling. The chemistry of starter batteries is generally lead-acid, with a configuration that supports quick, large current draws. These batteries are not intended for deep discharges and may suffer from reduced lifespan if used in such a manner. They are commonly found in automotive applications for starting combustion engines and in devices that require a rapid, powerful energy input.
Furthermore, deep cycle batteries generally require more maintenance, particularly flooded lead-acid types, which necessitate regular checking and maintenance of electrolyte levels, though AGM and gel batteries are considered low-maintenance. Starter batteries also require periodic maintenance for flooded types, but the demands are less frequent due to their less strenuous operational requirements. Cost-wise, deep cycle batteries tend to be more expensive due to their robust construction and the materials that allow for deep cycling, while starter batteries are generally more affordable, reflecting their simpler construction and intended use for less demanding applications.
In summary, the choice between a deep cycle battery and a normal battery hinges on the application's requirements: deep cycle batteries for applications needing prolonged, steady power output, and normal batteries for those requiring a quick, powerful burst of energy.
Sealed Lead Acid (SLA) deep cycle batteries typically offer a lifespan of 3 to 5 years, while with proper maintenance, Flooded lead-acid (FLA) deep cycle batteries can last up to 8 years. Absorbent Glass Mat (AGM) deep cycle batteries generally have a slightly longer lifespan, averaging about 5 to 7 years. Nickel-iron (NiFe) deep cycle batteries are known for their longevity, with a service life that can exceed 20 years. Lithium iron phosphate (LiFePO4) deep cycle batteries provide a long cycle life, often exceeding 10 years, and can endure thousands of cycles. It's important to note that the actual lifespan of a deep cycle battery can vary based on factors such as usage patterns, depth of discharge, charging regime, and environmental conditions.
Testing a deep cycle battery involves several steps to ensure its performance, safety, and longevity. Here's a general procedure you can follow, adapted from the principles outlined in various sources:
-Visual inspection: Start with a visual check for any physical damage, leaks, or swelling in the battery casing.
-Specific gravity test: Use a hydrometer to measure the specific gravity of the electrolyte in each cell of a lead-acid battery. This can indicate the state of charge and overall health of the battery.
-Voltage test: Check the open circuit voltage (OCV) of the battery. A fully charged deep cycle battery typically reads around 12.6 to 12.8 volts per cell for lead-acid types.
-Load test: Apply a load to the battery to simulate the discharge process. This can help determine the battery's ability to deliver power under load and its state of charge.
-Capacity test: This involves a controlled discharge of the battery to a specific voltage level (usually 10.5 to 11 volts for a 12-volt battery) and measuring the time it takes to reach that level. This test provides information about the battery's overall capacity.
-Charge test: After the capacity test, charge the battery to its maximum voltage and observe the time it takes to reach full charge. Monitor the charging current and voltage throughout the process.
-Cycling test: For a more thorough evaluation, perform a series of charge and discharge cycles, noting any changes in capacity, voltage, and overall performance.
-Temperature Monitoring: During testing, monitor the temperature of the battery, as excessive heat can be a sign of internal issues.
-Conductance test: This measures the internal resistance of the battery, providing insight into its health and expected lifespan.
-Insulation resistance test: Ensure that the insulation resistance is within the manufacturer's specifications to avoid short circuits or other issues.
-Use of testing equipment: Employ a battery analyzer or a battery testing system that can automate some of these tests and provide detailed readings and analysis.
-Safety precautions: Always follow safety guidelines during testing, including wearing protective gear and working in a well-ventilated area.
Remember that the testing procedure may vary depending on the battery type and the equipment you have available. For lithium-ion batteries, additional tests such as the incremental capacity analysis might be used to assess the battery's health. Always refer to the manufacturer's guidelines for the specific battery you are testing.

NEWARE has over 25 years of experience in battery testing, offering a variety of testing instruments and solutions not only for deep cycle batteries but also for other types of batteries such as button batteries, pouch batteries, cylindrical cells, and power cell batteries used in electric vehicles. We also provide customized testing solutions tailored to your specific needs. If you have any requirements for battery testing equipment or customized battery testing solutions, please feel free to contact us at any time.




The lab focuses on solid-state battery research to overcome traditional lithium batteries' safety and energy density issues, supporting environmental sustainability. It develops innovative solid-state electrolytes, refines electrode materials, and investigates ion transfer and interface stability to revolutionize battery technology.

The electric vehicle battery industry is rapidly developing, focusing on technological innovation, market competition, and sustainability. Research hotspots include solid-state batteries, new types of electrolytes, BMS optimization, and recycling technologies. The environmental adaptability, safety, and economic viability of batteries are key research areas, and the industry is expected to undergo more innovation and transformation.

Specializing in battery preparation technology research, the focus is on overcoming existing energy storage challenges by innovating in electrode materials, battery chemistry, and manufacturing processes to improve performance, safety, and reduce costs. Sustainability and recycling technologies for batteries are also emphasized to mitigate environmental impacts and foster the growth of green energy.