Since the 1970s. Lithium-ion batteries have attracted wide attention after researchers found the lithium storage performance of layered oxides and sulfides. Multi-application scenarios not only reflect the advantages of lithium batteries, but also the driving force of their development. As shown in Figure 1, lithium batteries only need to meet the operating temperature of 15~35 °C in applications such as electric vehicles and portable electronic devices. However, in some special application scenarios, lithium batteries are required to break through this temperature range. For example, the oil
industry needs lithium batteries to adapt to a working environment of about 80 °C, and lithium batteries for extreme environment working robots need to operate stably in an environment of ~150 °C; in terms of low temperature, polar exploration, aerospace industry and other application scenarios require lithium batteries to operate in an environment of -40 °C or even -80 °C. Such extreme operating temperatures pose challenges for the operation of lithium batteries.

Figure-1-Different-application-scenarios-of-battery
Lithium-ion battery with wide temperature range is a kind of lithium ion battery which can work normally and charge and discharge in a wide temperature range. However, lithium batteries with wide temperature range also face many challenges. As shown in Figure 2, too low operating temperature will slow down the kinetic process of electrochemical reaction in lithium batteries, which will cause the battery polarization to increase, the capacity to decrease, and even difficult to work. In addition, too low working temperature may also change the electrochemical reaction path. For example, at low temperature, Li+, which should be embedded in the interlayer of graphite anode, may be reduced on the surface of graphite anode to form dendrites, which endangers the safety of the battery. In the high temperature working environment, due to the decrease of the stability of the electrolyte/electrode interface, it is impossible to prevent the occurrence of side reactions between the electrode and the electrolyte. Therefore, the main challenge faced by lithium batteries comes from too many side reactions.
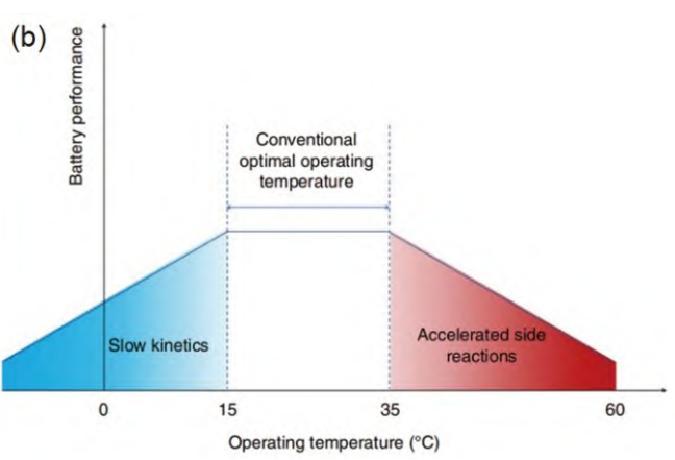
Figure-2-Relationship-between-working-temperature-and-battery-performance
Extreme temperature conditions will have a series of adverse effects on battery performance. Among them, extreme temperature conditions mainly affect the selection of electrolytes and the electrode / electrolyte interface.
1.Selection of solvents and electrolyte salts: LiPF6 currently used in commercial electrolytes has the advantages of high conductivity, compatibility with graphite anodes, and good thermal stability. However, LiPF6 is very easy to decompose with water: LiPF6+H2O↔LiF+2HF+POF3 and PF5+H2O↔2HF+POF3; at high temperature, the decomposition reaction of LiPF6 is more likely to occur. Therefore, it is necessary to consider some high temperature stable lithium salts, such as LiBOB, LiDFBOB, LiFSI, LiTFSI and so on.
In addition, the selection of organic solvents has a great influence on the high and low temperature characteristics of lithium battery electrolyte. For lithium batteries working at extreme temperatures, the electrolyte solvent also needs to meet the requirements of low melting point, high boiling point and high flash point to ensure the wide operating temperature range and safety of the electrolyte. But the choice of solvent is more complex, need specific analysis.
2.Affect the electrode/electrolyte interface: This is mainly due to the fact that when the temperature is too high, side reactions such as the decomposition of the electrolyte are more likely to occur, and when the temperature is low, there will also be safety hazards such as lithium dendrites. The fundamental impact on the electrode/electrolyte interface should be attributed to the different reactions between the electrode and different electrolytes in a wide temperature range.
The research progress of wide temperature range batteries will mainly focus on the electrolyte research of low temperature LIBs, high temperature LIBs and wide temperature LIBs:
1.Research progress of electrolytes for low-temperature lithium-ion batteries: At low temperatures, the freezing of the electrolyte and the precipitation of lithium salts affect the migration, kinetics and coulombic efficiency of Li+. In addition, the Li+ desolvation process through the electrode-electrolyte interface becomes more challenging at low temperatures. In response to this problem, there are mainly the following strategies:
(1)Low melting point solvents: In previous studies, the use of solvents with medium dielectric constant and low freezing point is a common strategy. Some carbonate solvents such as ethyl-2,2,2-trifluoro-ethyl carbonate can make lithium batteries work at −20 °C, while some carboxylate solvents such as ethyl acetate or methyl butyrate have lower freezing points, which can make lithium batteries adapt to cold environments below −30 °C.
(2)Weakly solvated solvent system: With the decrease of temperature, the desolvation potential of Li+ at the electrode interface increases exponentially. The introduction of weakly solvated solvent in the electrolyte can change the reaction kinetics and migration rate of Li+ at the interface between the electrolyte and the electrode. For example, solvents such as 1,3-dioxane and DEE can be introduced. At the same time, 'inert' diluents can also be added to high-concentration salt electrolytes, which can change the Li+ transport path and interaction with other molecules, thereby reducing the desolvation energy of Li+, such as adding BTFE diluents.
(3)Interface film: The energy barrier of Li+ transport at the electrode interface is mainly determined by the structure of solvation sheath and SEI layer. The desolvation process of Li+ occurs after the formation of SEI film, and its migration through SEI usually requires higher activation energy, which is also the main kinetic obstacle of Li+ transport at low temperature. The construction of a stable and highly conductive SEI is crucial to improve the performance of LIBs at low temperatures, but the regulation of SEI is more complicated and will not be introduced here.
2.Research progress of electrolytes for high-temperature lithium-ion batteries: In traditional non-aqueous organic electrolytes, the electrolyte will decompose when it exceeds 55 °C, resulting in battery expansion, poor interface contact, and increased internal resistance. Therefore, the introduction of thermally stable lithium salts and solvents to construct a stable electrolyte membrane, and the use of solid electrolytes and temperature-responsive electrolytes are very important for improving the high-temperature performance of LIBs.
(1)Lithium salts: LiTFSI, LiFSI, LiBOB, LiDFOB and LiBF4 are used in high temperature LIBs due to their excellent thermal stability compared to commercial LiPF6, which is easy to decompose at high temperature. It is worth emphasizing that when LiTFSI and LiFSI are used alone, they will corrode the aluminum foil due to the F-S bond in the anion or the residual Cl- in the synthesis process, but when used in combination with LiBOB, LiDFOB or LiBF4, they can reduce the corrosion of the aluminum foil.
(2)Solvent: The properties and solvation structure of the solvent determine the operating temperature of the battery and the migration performance of Li+. Adjusting a suitable thermally stable solvent becomes a necessary condition for high-temperature electrolyte design. So far, researchers usually use fluorinated carbonates, sulfite/nitrile solvents, and ionic liquids as solvents for high-temperature lithium-ion batteries.
(3)Interface film: The interface layer between the electrolyte and the electrode affects the rate, cycle life, internal resistance and safety performance of the battery. At present, 1,3-propyl sultone, vinyl sulfate, tripropyl phosphate, etc.are considered to be effective additives for stabilizing CEI.
(4)Solid electrolyte: In order to further expand the operating temperature of LIBs and avoid thermal runaway, solid electrolyte is a good choice.
3.Electrolyte for wide-temperature lithium-ion batteries: For wide-temperature electrolytes, the effects of high and low temperatures on the salt, solvent and interface properties of the electrolyte need to be fully considered in the composition and functional design of the electrolyte. So far, there are mainly the following strategies:
(1)Mixed lithium salts: Considering the thermal instability of traditional LiPF6 salts at high temperatures, the mixing of several lithium salts is an effective method to prolong the working temperature of LiPF6 salts. The combination of various lithium salts can make up for each other 's deficiencies and produce synergistic effects, such as : LiBF4+LiBOB (+MB, -40 ~ 65 °C ), LiTFSI+LiDFOB (+AND/EC, -40 °C ~ 150 °C ).
(2)High stability solvent: In order to broaden the working temperature range of the electrolyte, the wide liquid range solvent has attracted much attention due to its heat resistance and low freezing point. However, a single wide liquid range solvent is relatively rare. Generally, a combination of low freezing point and heat-resistant solvents is used to improve the liquid range of the electrolyte. Low freezing point solvents include ethyl acetate, methyl propionate, dichloromethane and liquefied petroleum gas. Heat-resistant solvents include esters, nitriles, sulfones, etc. In addition to the above solvents, researchers have also developed some new solvents to improve the performance of electrolytes.
(3)Additives: The main purpose of adding additives is to improve the performance of the formed CEI/SEI. The types of additives are wide and the effects are different.
(4)Ionic liquid.
(5)Solid state eletrolyte.
Figure 3 is a common electrolyte design for wide temperature range batteries.
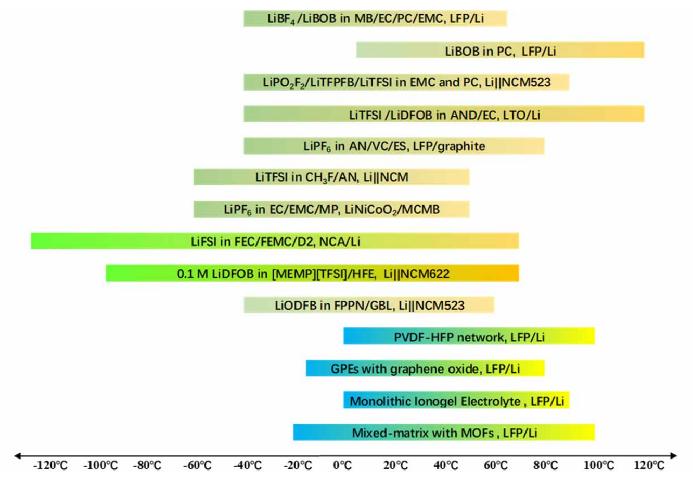
Figure-3-Common-electrolyte-design-for-wide-temperature-range-batteries
1.Low temperature lithium ion battery: The researchers chose 1.0 M lithium bis(fluorosulfonyl) imide (LiFSI) in tetrahydrofuran (THF) as the reference electrolyte because of its low viscosity, high ionic conductivity, low freezing point and good compatibility with lithium metal anodes at low temperatures. Subsequently, the composite electrolyte was formed by adding additive-grade perfluorinated surfactant NaPFO to the reference electrolyte. By using this electrolyte, Li+ can be rapidly transported on the bulk phase and interface, thereby achieving a stable cycle and high power output of the ultra-low temperature LMB (Figure 4).
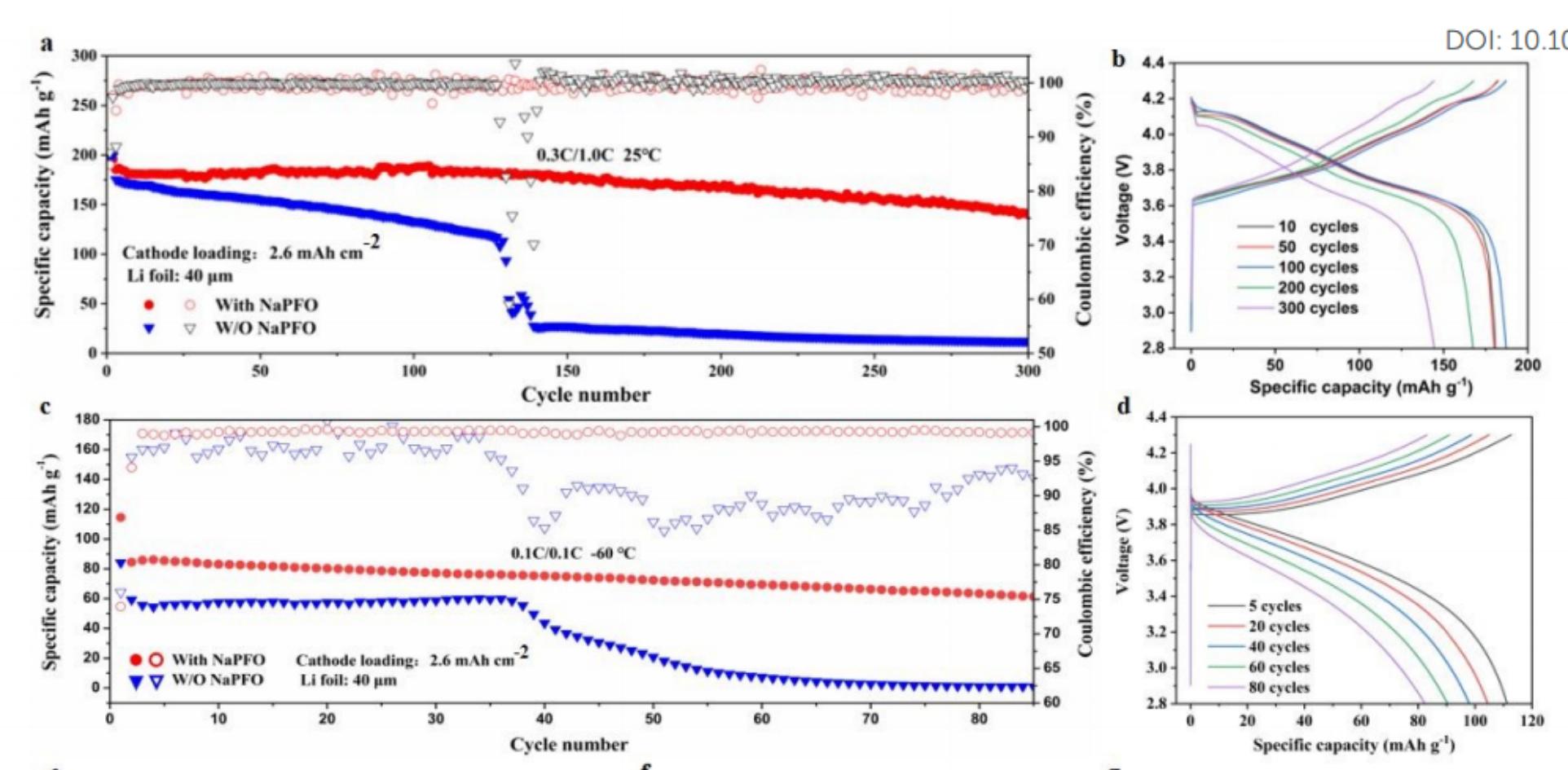
Figure-4-The-performance-of-Li||NMC811-full-battery-under-benign-and-ultra-low-temperature-conditions
2.High temperature lithium ion battery: The researchers used glycerol triacetate (GTA) as a new type of flame retardant high temperature electrolyte solvent, with GTA, EC and FEC as cosolvents, LiTFSI and LiDFOB as lithium salts to prepare high temperature electrolyte. Subsequently, the performance of the battery was verified at 100 °C with different cathode materials. The results show (Figure 5) that using this electrolyte, the performance of the battery is improved and the safety is enhanced even at extreme temperatures (100 ° C).
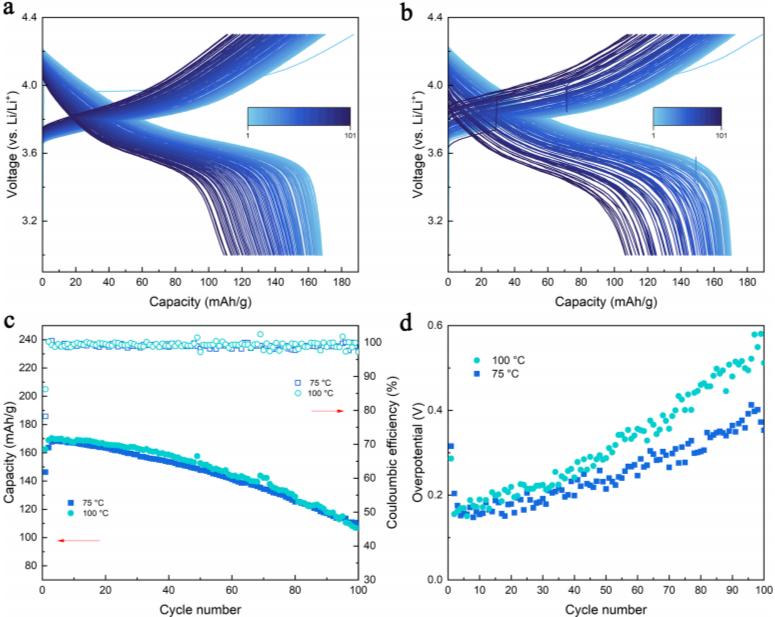
Figure-5-The-cycle-performance-of-Li/NCM523-battery-at-different-temperatures
NEWARE
47690 Westinghouse Dr, Fremont, CA 94539




● Voltage&Current Accuracy:±0.01% F.S.
● Recording Frequency:100Hz
● Current Response Time:≤1ms
● Minimum Pulse Width:500ms
● Off-Line Test:1GB/CH
● Cycle Life, GITT Test, DCIR Test, dQ/dV Curve

● Voltage & Current Accuracy:±0.01% F.S.
● Recording Frequency:10Hz
● Sampling Time:100ms
● Current Response Time:≤1ms
● Minimum Pulse Width:500ms
● Off-Line Test: 1GB

● Voltage & Current Accuracy:±0.05% F.S.
● Recording Frequency:10Hz
● Sampling Time:100ms
● Current Response Time:≤1ms
● Energy Efficiency:>65%
● Off-Line Test: 1GB

● Voltage & Current Accuracy:±0.05% F.S.
● Recording Frequency:10Hz
● Sampling Time:100ms
● Current Response Time:≤1ms
● Energy Efficiency:>65%
● Off-Line Test: 1GB

● Voltage & Current Accuracy:±0.05% F.S.
● Recording Frequency:10Hz
● Sampling Time:100ms
● Current Response Time:≤1ms
● Energy Efficiency:>65%
● Off-Line Test: 1GB

● Voltage Accuracy:±0.02% F.S.
● Current Accuracy:±0.05% F.S.
● Resolution Ratio AD/DA:16bit
● Current Response Time:≤1ms
● Minimum Pulse Width:100ms
● Off-Line Test:1GB/CH

● Voltage & Current Accuracy:±0.05% F.S.
● Recording Frequency:100Hz
● Current Conversion Time:≤6ms
● Current Response Time:≤3ms
● Minimum Pulse Width:100ms
● Feedback Efficiency (Max) :75%

● Voltage & Current Accuracy:±0.02% F.S.
● Voltage & Current Stability:±0.01% F.S.
● Recording Frequency:1000Hz
● Resolution AD:16bit
● Current Response Time:≤100μs
● Off-Line Test: 1GB